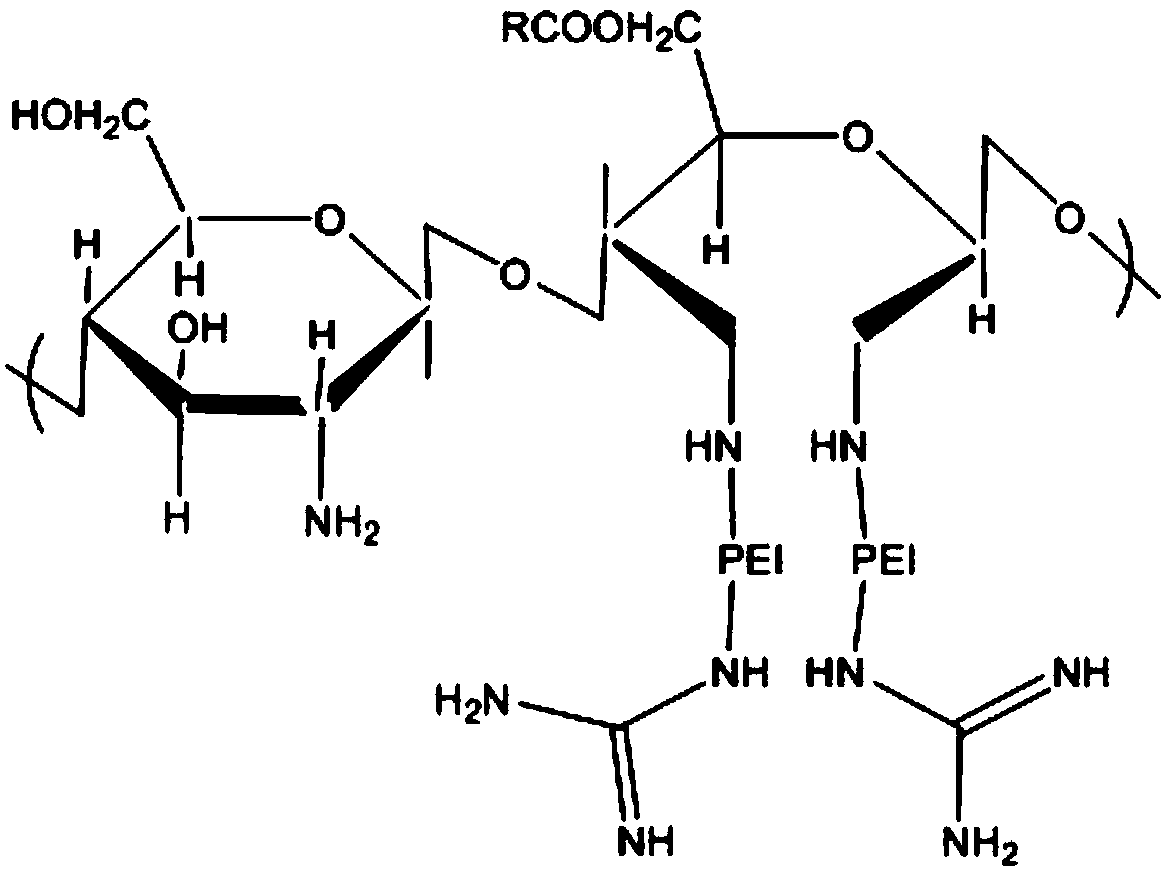
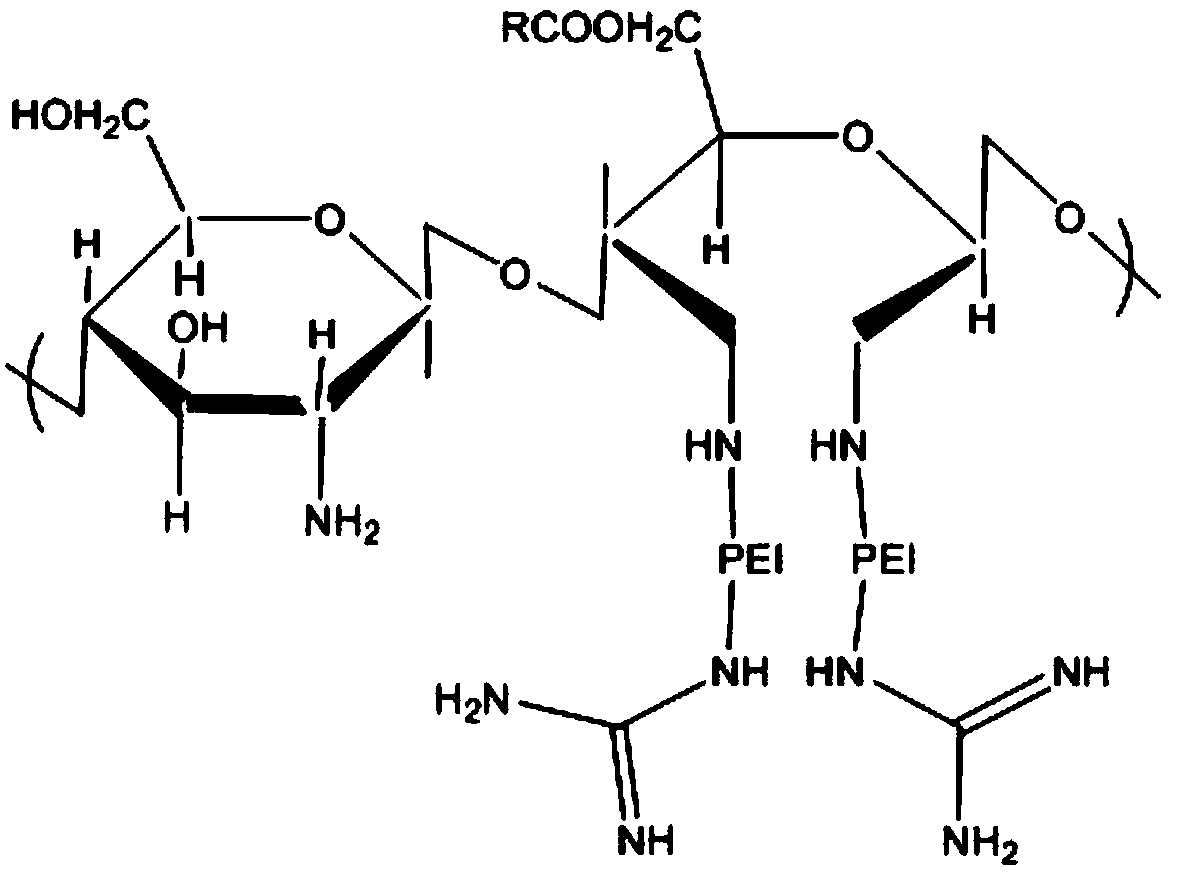
Amphiphilic derivative of oil acylated chitosan oligosaccharide guanidine grafted polyethyleneimine and preparation method thereof
A technology of oleoylated chitosan and polyethylenimine, which is applied in the field of biomedical materials, can solve the problems of non-degradability, poor solubility of chitosan, and inability to fully utilize the advantages of chitosan, and achieve low cytotoxicity, Effect of High Transfection Efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
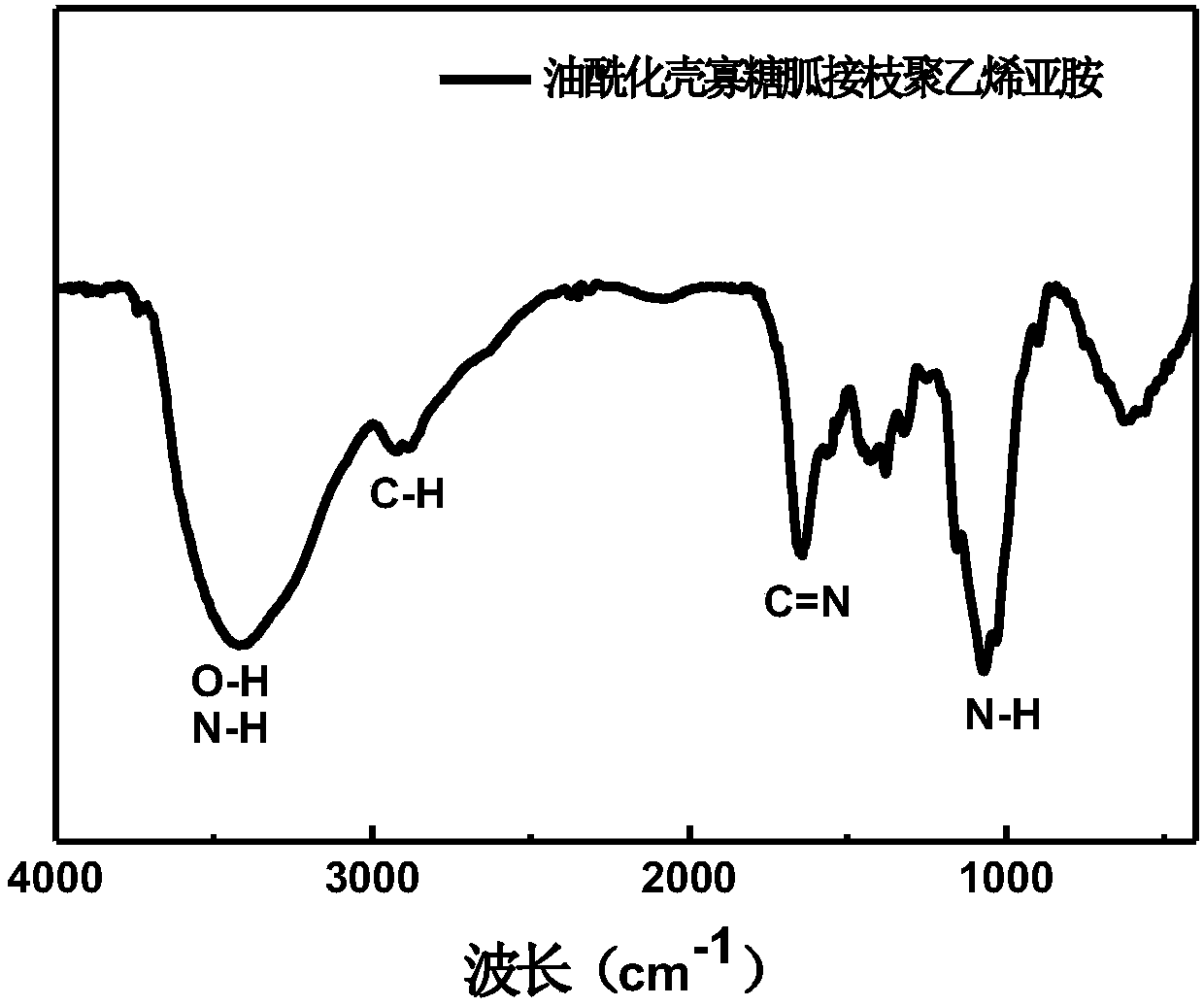
[0035] 1) Dissolve (MW=1.5kDa) chitosan oligosaccharide in (0.02wt%, pH4.5) sodium acetate buffer, add 70wt% potassium periodate aqueous solution, and react at 4°C for 48h. After dialysis and lyophilization, oxidized chitosan (OXCOS) was obtained.
[0036] 2) Add 5 times the number of moles of chitosan oligosaccharide structural units (MW=0.8kDa) polyethyleneimine to the above aqueous solution, and react for 48 hours at -10 degrees Celsius in the dark, and add sodium tetrahydroborate to continue the reaction for 24 hours. The product is purified and lyophilized to obtain polyethyleneimine grafted chitosan (COS-PEI).
[0037] 3) Add CS-PEI to 1H-pyrazole-1-carboxamidine hydrochloride 1.5 times the moles of polyethyleneimine and N, N'1.5 times the moles of polyethyleneimine at 25°C -Reaction in diisopropylethylamine aqueous solution for 24h. The product was purified by dialysis and lyophilized to obtain COSG-PEI white solid.
[0038] 4) Add COSG-PEI and methanesulfonic acid into the...
Embodiment 2
[0040] 1) Dissolve (MW=3.0kDa) chitooligosaccharides in (0.05%, pH 5) sodium acetate buffer, add 80wt% potassium periodate, and react at 10°C for 60 hours. After dialysis and lyophilization, oxidized chitosan (OXCOS) was obtained.
[0041] 2) Add 6 times the moles of chitosan oligosaccharide structural unit (2.0kDa) polyethyleneimine to the above aqueous solution, react at -10 degrees Celsius and avoid light for 60 hours, add sodium tetrahydroborate and continue the reaction for 48 hours, and the product is purified After freeze-drying, polyethylenimine grafted chitosan oligosaccharide (COS-PEI) was obtained.
[0042] 3) At 35℃, add CS-PEI to 1H-pyrazole-1-carboxamidine hydrochloride 2 times the moles of polyethyleneimine and N, N'2 times the moles of polyethyleneimine -Reaction in diisopropylethylamine aqueous solution for 48h. The product was purified by dialysis and lyophilized to obtain COSG-PEI white solid.
[0043] 4) Add COSG-PEI and methanesulfonic acid to the four-neck fl...
Embodiment 3
[0045] 1) Dissolve ((MW=2.0kDa) chitosan oligosaccharides in (0.08wt%, pH 3.5) sodium acetate buffer, add 50wt% potassium periodate aqueous solution, and react at 15°C for 36 hours. Lyophilized by dialysis Then get oxidized chitosan (OXCOS).
[0046] 2) Add 3 times the mole number of chitosan oligosaccharide structural unit (1.5kDa) polyethyleneimine to the above aqueous solution, react for 36h at -10 degrees Celsius and avoid light, add sodium tetrahydroborate to continue the reaction for 36h, and the product is purified After lyophilization, polyethylenimine grafted chitosan oligosaccharide (COS-PEI) was obtained.
[0047] 3) Add CS-PEI to 1H-pyrazole-1-carboxamidine hydrochloride 3 times the moles of polyethyleneimine and N, N'3 times the moles of polyethyleneimine at 20°C -React 36h in diisopropylethylamine aqueous solution. The product was purified by dialysis and lyophilized to obtain COSG-PEI white solid.
[0048] (4) Add COSG-PEI and methanesulfonic acid to the four-neck f...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com