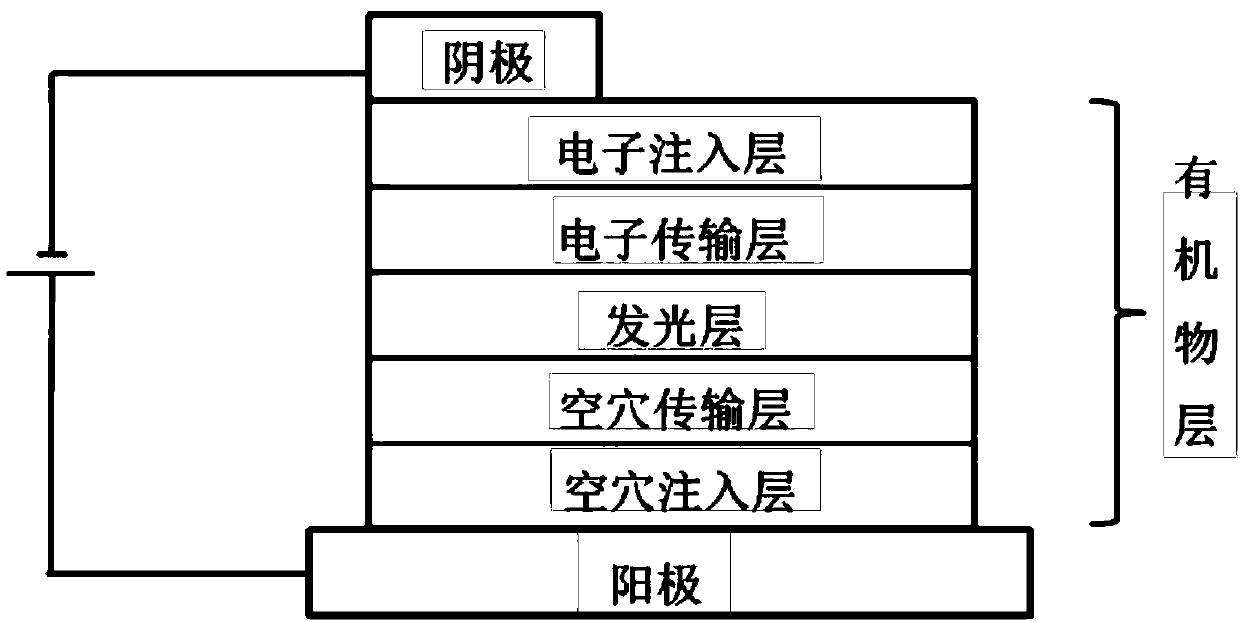
Aryl amine derivative containing carbazole and carboline and organic electroluminescent element containing aryl amine derivative
An arylamine and derivative technology, applied in the field of novel arylamine derivatives, can solve the problems of difficult to use organic light-emitting elements, and achieve the effect of improving lifespan
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0072] Embodiment 1: Synthesis of compound 3-1 (DC1A-md-C3)
[0073] The synthesis route of DC1A-md-C3 is shown below.
[0074]
[0075] Synthesis of Intermediate 1:
[0076] In a 250ml two-necked flask, add 7g (42mmol) of δ-carboline (δ-carboline), 9.3g (46mmol) of 1-bromo-3-nitrobenzene (1-bromo-3-nitrobenzene), 0.52g (3mmol) of CuI, 0.94g (5mmol) of 1,10-phenanthroline (1,10-phenanthroline), 22g (104mmol) of K 3 PO 4 , 120ml of two Dioxane, reflux at 80-100°C for 24 hours in a nitrogen atmosphere. After the reaction, the reactant was dissolved in dichloromethane (Methylene Chloride, MC) and filtered with diatomaceous earth (Celite). After the filtered solution was distilled under reduced pressure, extraction was performed with MC and distilled water. The extracted MC layer was washed with MgSO 4 After removing water, the solvent was distilled off under reduced pressure to obtain a yellow solid. From this solid, a white solid (1) (7.5 g, yield: 65%) was isolated ...
Embodiment 2
[0082] Embodiment 2: Synthesis of Compound 3-2 (C1A-md-DC3)
[0083] The synthesis route of C1A-md-DC3 is shown below.
[0084]
[0085] Synthesis of Intermediate 3:
[0086] In a 250ml two-necked flask, add 15g (89mmol) of carboline (δ-carboline), 37.8g (134mmol) of 1-bromo-3-iodobenzene (1-bromo-3-iodobenzene), 1.1g ( 5.79mmol) of CuI, 2g (11.13mmol) of 1,10-phenanthroline (1,10-phenanthroline), 47g (220mmol) of K 3 PO 4 , 300ml of two Dioxane, reflux at 80-100°C for 24 hours in a nitrogen atmosphere. After the reaction, the reactant was dissolved in MC and filtered through diatomaceous earth (Celite). The filtered solution was distilled under reduced pressure, and extracted with dichloromethane and distilled water. The extracted dichloromethane layer was washed with MgSO 4 After removing water, the solvent was distilled off under reduced pressure to obtain a yellow solid. From this solid, a white solid (3) (18.0 g, yield: 62%) was isolated by column chromatograp...
Embodiment 3
[0090] Embodiment 3: the synthesis of compound 3-3 (C3A-md-DC3)
[0091] The synthesis route of C3A-md-DC3 is shown below.
[0092]
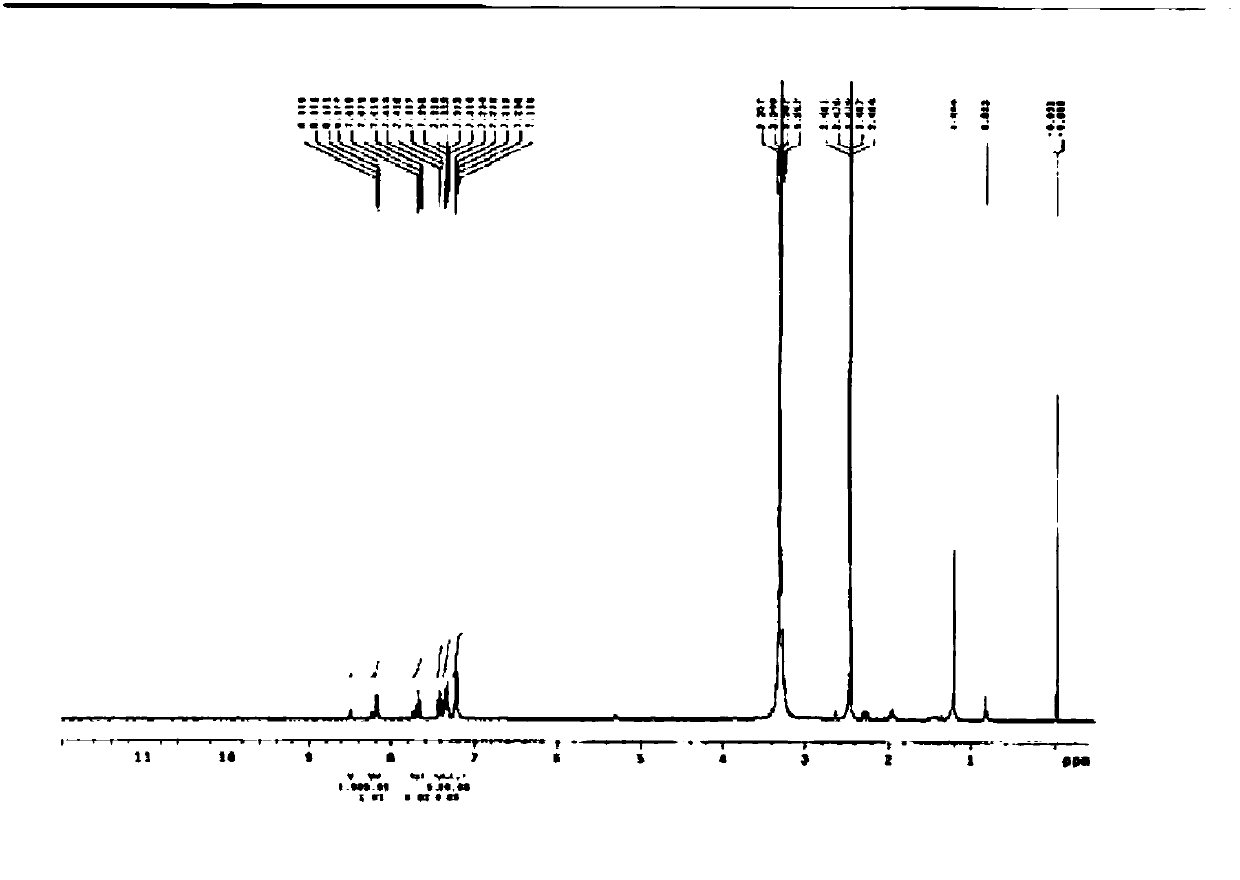
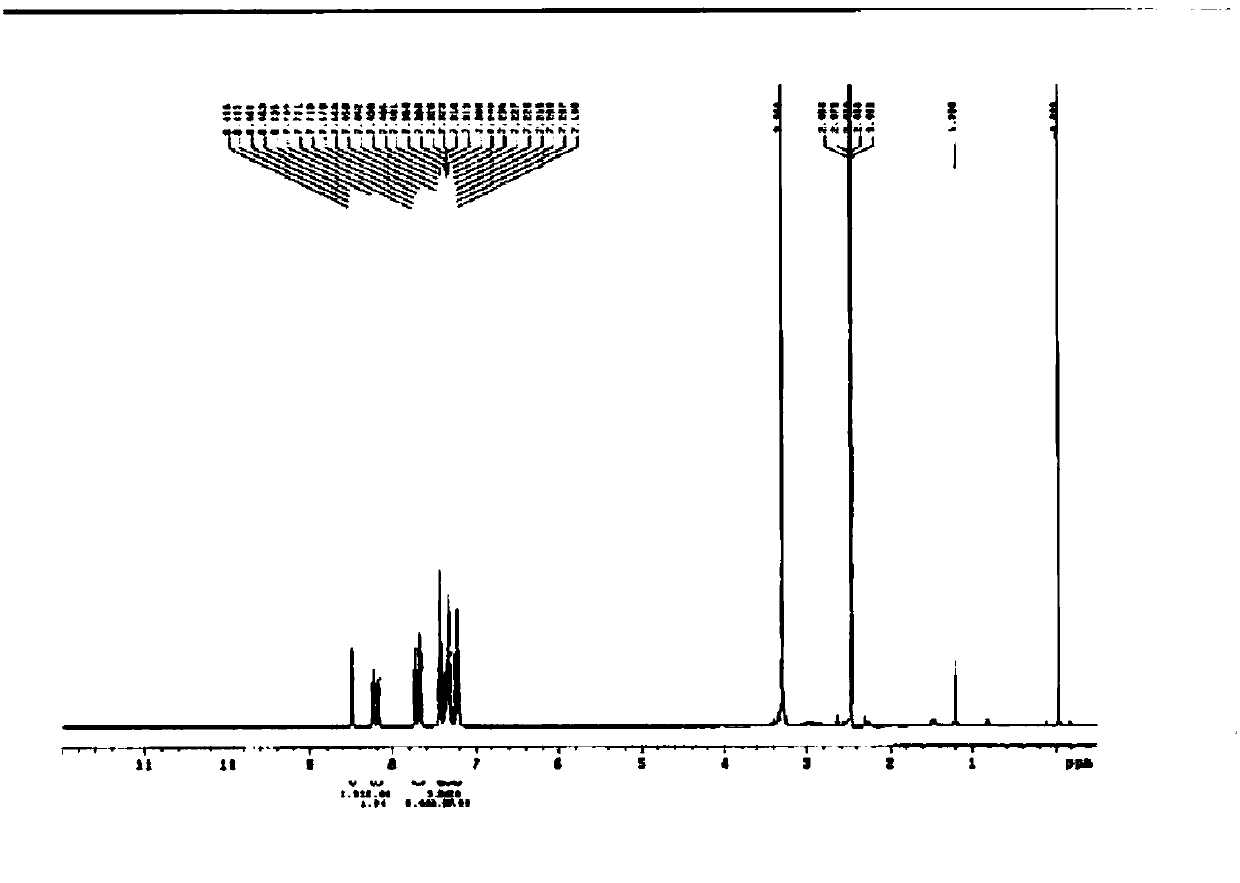
[0093] In a 250ml two-necked flask, 0.6g (2.3mmol) of Intermediate 2, 1.7g (5mmol) of Intermediate 3, 0.67g (6.9mmol) of NaO t Bu was added into 100 ml of toluene, stirred in a nitrogen atmosphere, and the reaction temperature was raised to 80° C. and reacted for 1 hour. Add 0.07g (0.07mmol) of Pd to it 2 (dba) 3 , 0.02g (0.09mmol) of (tert-Bu) 3 P, react at 80°C for 4-5 hours. The above reaction liquid was filtered to remove the salt. The filtrate was distilled off to remove toluene, and the concentrate was separated with a column to obtain 1.1 g of compound 3-3 (C3A-md-DC3) (yield: 68%). The NMR analysis result of the obtained compound 3-3 (C3A-md-DC3) is as follows (with reference to Figure 4 ).
[0094] NMR analysis results: 1 H NMR(300MHz,DMSO)δ8.54(m,3H),8.28(d,3H),7.75(m,3H),7.65(m,12H),7.54(m,3H),7.44(m,6H) ,7.36(m,3H)
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com