Saddle-shaped perylene diimide tetramer, and preparation method and application thereof
A peryleneimide tetramer and saddle-shaped technology, which is applied in the field of saddle-shaped peryleneimide tetramer and its preparation, can solve the problems of low photoelectric conversion efficiency and easy aggregation of peryleneimide derivatives, and achieve Effects of preventing close packing, improving performance, and suppressing crystallinity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
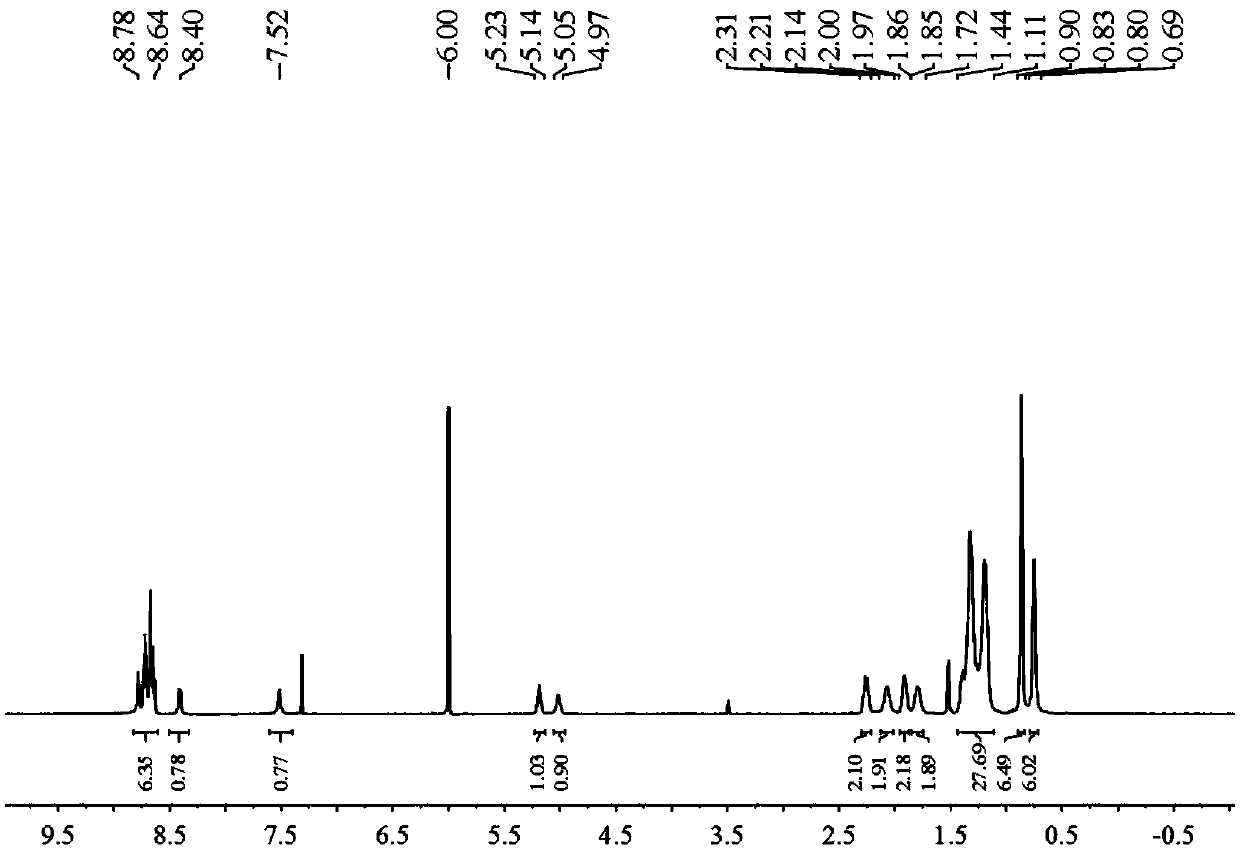
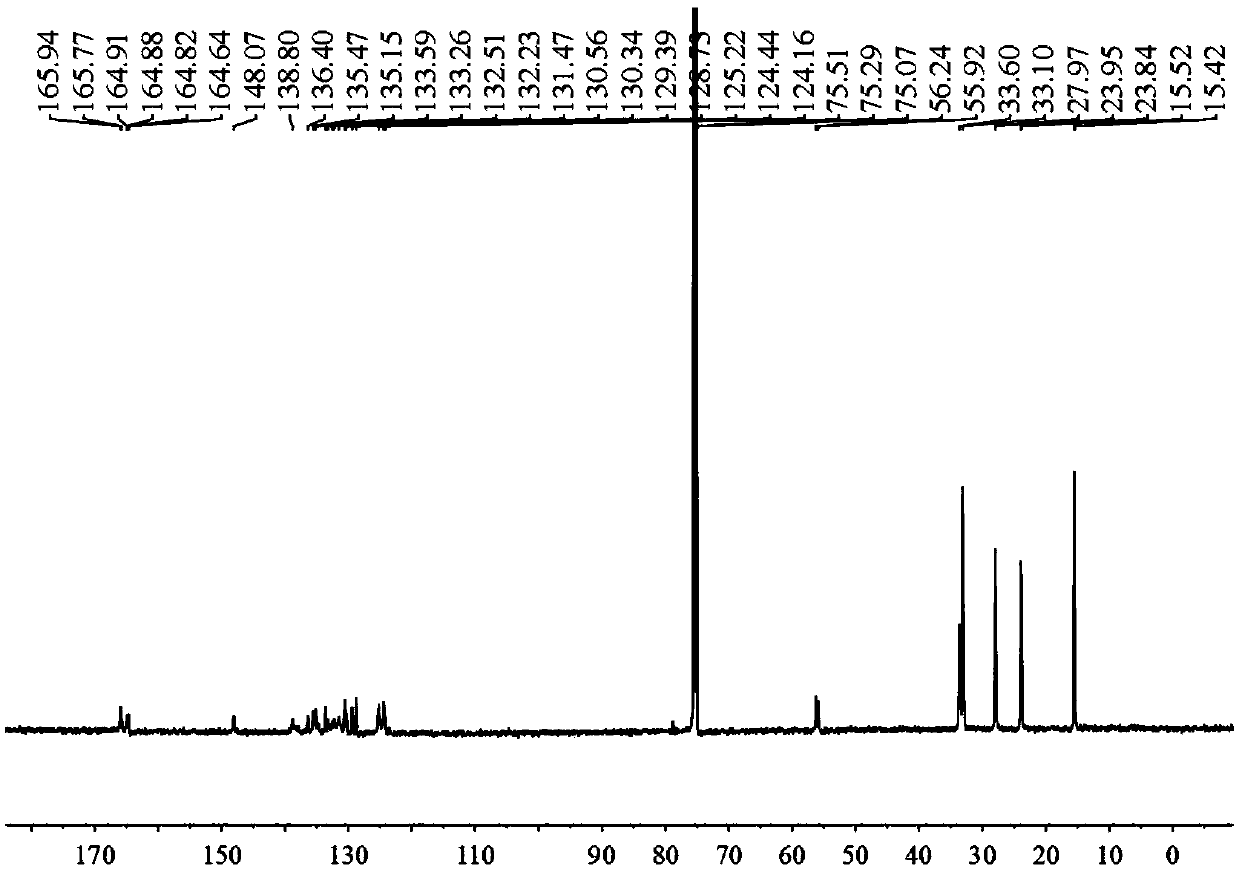
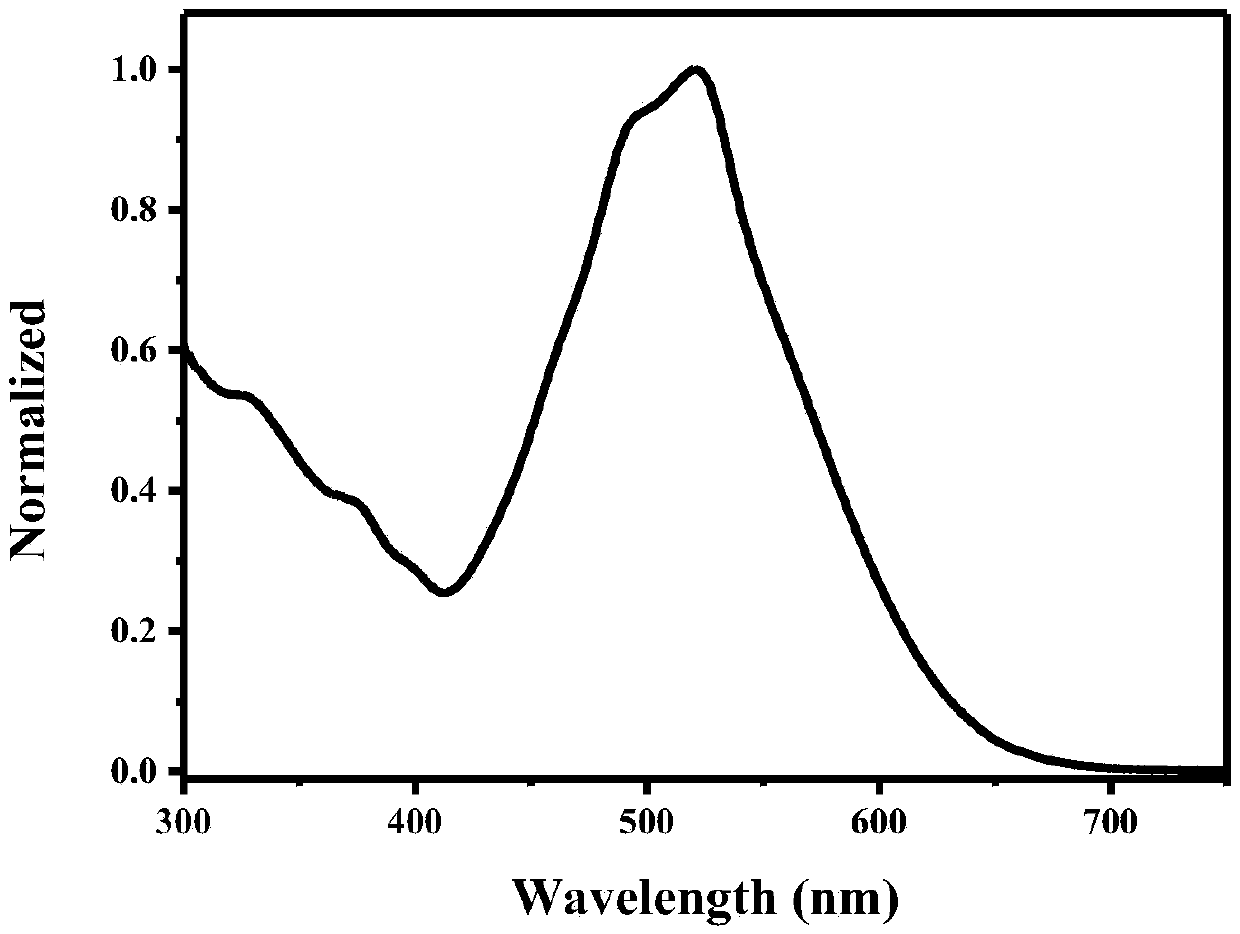
Examples
Embodiment 1
[0046] (1) Preparation of intermediate compound (Ⅳ)
[0047] The compound (225mg, 0.34mmol, 1.0eq) of the structure shown in formula III was added to 100mL Schlenk, vacuum-dried for 0.5h, during which the argon gas was changed 3 times, 30mL tetrahydrofuran was added, and under the protection of Ar gas at -78°C, drop Add n-BuLi (1.4mL, 2.5M in hexane, 3.49mmol, 10.0eq), slowly raise the temperature to 60°C, and react for 3h; add Sn(C 4 h 9 ) 3 Cl (0.76mL, 2.8mmol, 8.0eq), slowly warmed up to room temperature, and reacted overnight. Add 1mLCH at -78°C 3 OH quenches the reaction. Transfer the reaction system to a 250mL separatory funnel, separate the aqueous phase, and use 3×15mL CH 2 Cl 2 The aqueous phase is extracted and the organic phases are combined. The organic phase was washed with 3 x 15 mL of water and washed with anhydrous MgSO 4 After drying, filtering, and removing the solvent, 1.131 g of compound (IV) was obtained.
[0048]
[0049] (2) Preparation of sa...
Embodiment 2
[0059] (1) Preparation of intermediate compound formula (Ⅳ)
[0060] Add the compound (226mg, 0.34mmol, 1.0eq) of the structure shown in formula III-B into 100mL Schlenk, vacuum-dry for 0.5h, change nitrogen 3 times during this period, add 30mL tetrahydrofuran, and drop Add t-BuLi (1.12mL, 2.5M in hexane, 2.79mmol, 8.0eq), slowly raise the temperature to 50°C, and react for 3h; add Sn(C 4 h 9 ) 3 Cl (0.57mL, 2.1mmol, 6.0eq), slowly warmed up to room temperature, and reacted overnight. The reaction was quenched by adding 1 mL of saturated sodium bicarbonate solution at -90°C. Transfer the reaction system to a 250mL separatory funnel, separate the aqueous phase, and wash with 3×15mL CHCl 3 The aqueous phase is extracted and the organic phases are combined. The organic phase was washed with 3 x 15 mL of water and washed with anhydrous MgSO 4 Dry, filter, and remove the solvent to obtain 1.131 g of compound (IV-B).
[0061]
[0062] (2) Preparation of saddle type perylen...
Embodiment 3
[0072] (1) Preparation of intermediate compound (Ⅳ)
[0073] Add the compound (225mg, 0.34mmol, 1.0eq) of the structure shown in formula III into 100mL Schlenk, vacuum-dry for 0.5h, change helium 3 times during this period, add 30mL tetrahydrofuran, -60℃ under the protection of helium, drop Add n-BuLi (1.68mL, 2.5M in hexane, 4.19mmol, 12.0eq), slowly raise the temperature to 70°C, and react for 3h; add Sn(C 4 h 9 ) 3 Cl (0.95mL, 3.5mmol, 10.0eq), slowly warmed up to room temperature, and reacted overnight. Add 1mL CH at -60°C 3 OH quenches the reaction. Transfer the reaction system to a 250mL separatory funnel, separate the aqueous phase, and use 3×15mL CH 2 Cl 2 The aqueous phase is extracted and the organic phases are combined. The organic phase was washed with 3 x 15 mL of water and washed with anhydrous MgSO 4 After drying, filtering, and removing the solvent, 1.131 g of compound (IV) was obtained.
[0074]
[0075] (2) Preparation of saddle-shaped perylene im...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com