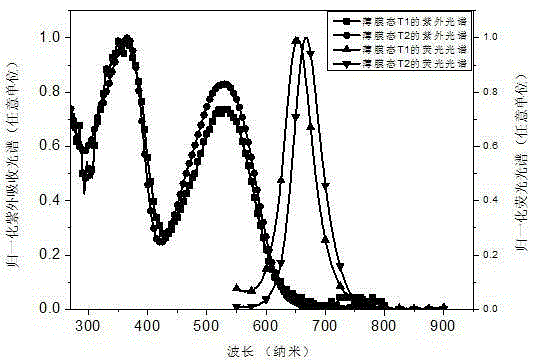
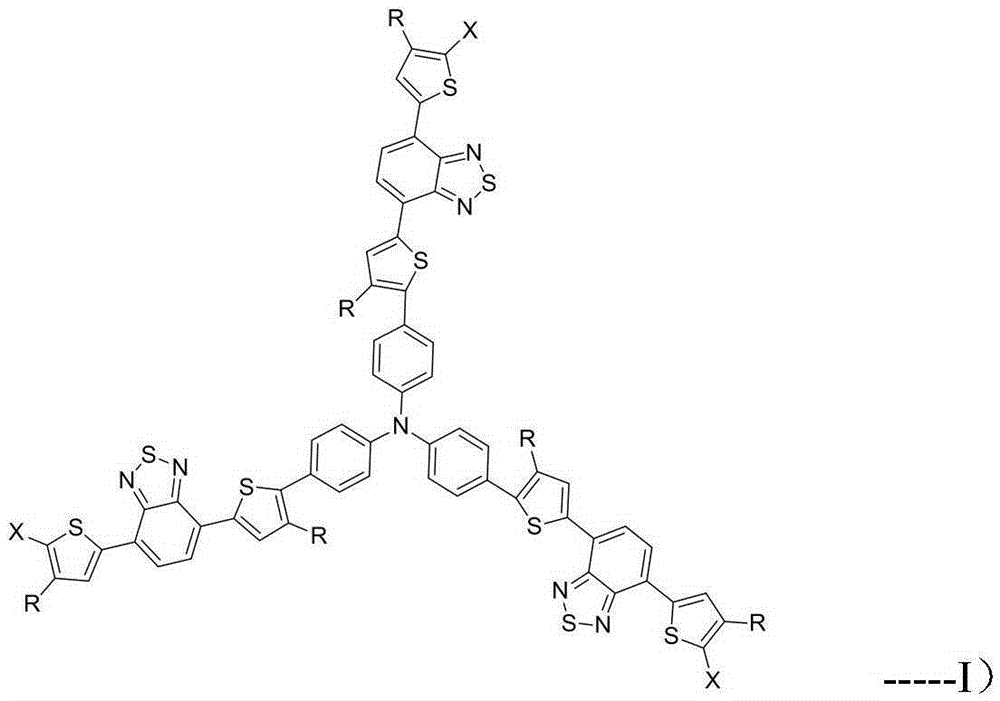
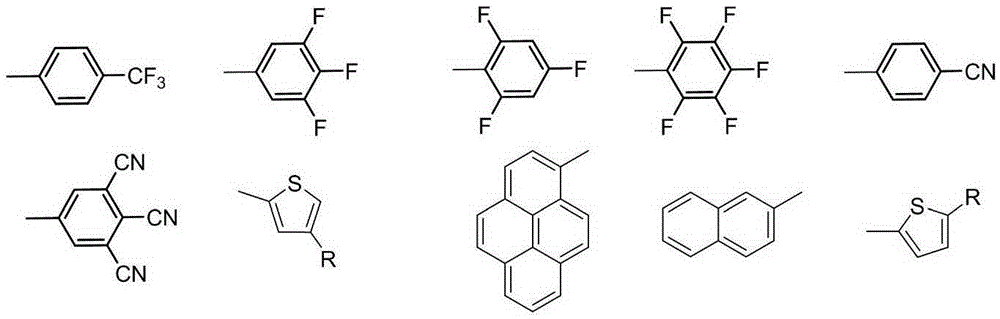
A kind of red light material and its preparation method and application
A red light and reaction technology, applied in luminescent materials, chemical instruments and methods, semiconductor/solid-state device manufacturing, etc., can solve problems such as the reduction of fluorescence quantum yield, and achieve reduced quenching effect, good fluorescence quantum efficiency, high efficiency and stability The effect of red light emission
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033]
[0034] Target compound T1
[0035] 【Reaction route】
[0036]
[0037] According to the reaction scheme, compound 1 and 4-(4-hexylthiophen-2-yl)-7-(5-bromo-4-hexylthiophen-2-yl)benzothiadiazole were catalysts Compound 2 was prepared by Suzuki coupling reaction under the conditions of , and NBS dissolved in DMF was slowly added dropwise in ice bath and protected from light to obtain compound 3 . Finally, the target compound T1 was prepared by Suzuki coupling reaction between 3 and boronate of pyrene under the condition of tetrakistriphenylphosphopalladium as catalyst. The specific steps of the Suzuki coupling reaction are as follows: in a 50 mL two-necked round-bottomed flask with a magnetic stirrer, sequentially add compound 13 (0.40 g, 0.21 mmol), pyrene borate (0.32 g, 0.96 mmol) and Pd(pph 3 ) 4 (10mg), vacuum three times for nitrogen. Add deoxygenated toluene (20mL), and then add K 2 CO 3 aqueous solution (2M, 8 mL). Turn on the condensed water, heat ...
Embodiment 2
[0039]
[0040] Target compound T2
[0041] 【Reaction route】
[0042]
[0043] According to the reaction scheme, 3 and 2-naphthylboronic acid undergo Suzuki coupling reaction under the condition of tetrakistriphenylphosphopalladium as a catalyst to obtain the target compound T2. The specific steps are: under the condition of avoiding light, in a 50mL two-necked round bottom flask with a magnetic stirrer, sequentially add compound 13 (0.30g, 0.16mmol), 2-naphthylboronic acid (0.14g, 0.72mmol), Pd (pph 3 ) 4(10mg), vacuum three times for nitrogen. Add deoxygenated toluene (20mL), and then add K 2 CO 3 aqueous solution (2M, 8 mL). Turn on the condensed water, heat to reflux at 95°C, and react for 48h. Extract with dichloromethane and water, dry the organic phase with anhydrous magnesium sulfate, filter with suction, and spin dry the organic phase. The eluent of column chromatography was dichloromethane and petroleum ether (1:3 by volume), and 0.164 g of the target pr...
Embodiment 3
[0045]
[0046] Target compound T3
[0047] 【Reaction route】
[0048]
[0049] According to the reaction scheme, 3 and p-trifluoromethylphenylboronic acid were subjected to Suzuki coupling reaction under the condition of tetrakistriphenylphosphopalladium as a catalyst to obtain the target compound T3. The specific reaction steps are: under dark conditions, in a 50mL two-necked round-bottomed flask with a magnetic stirrer, sequentially add compound 13 (0.30g, 0.16mmol), p-trifluoromethylphenylboronic acid (0.14g, 0.72mmol), Pd(pph 3 ) 4 (10mg), vacuum three times for nitrogen. Add deoxygenated toluene (20mL), and then add K 2 CO 3 aqueous solution (2M, 8 mL). Turn on the condensed water, heat to reflux at 95°C, and react for 48h. Extract with dichloromethane and water, dry the organic phase with anhydrous magnesium sulfate, filter with suction, and spin dry the organic phase. The eluent of column chromatography was dichloromethane and petroleum ether (1:3 volume r...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com