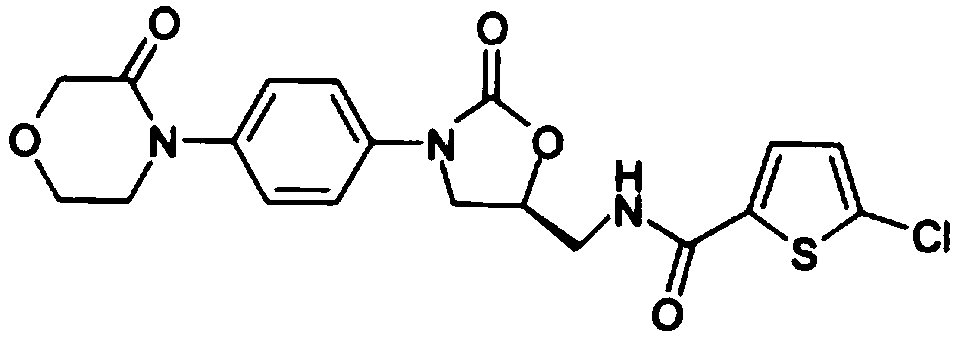
Rivaroxaban gastric retention tablets and preparation method thereof
A technology for rivaroxaban and gastric retention, which is applied in the field of medicine, can solve difficult problems, and achieve the effects of small side effects, stable retention effect, and simple preparation method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
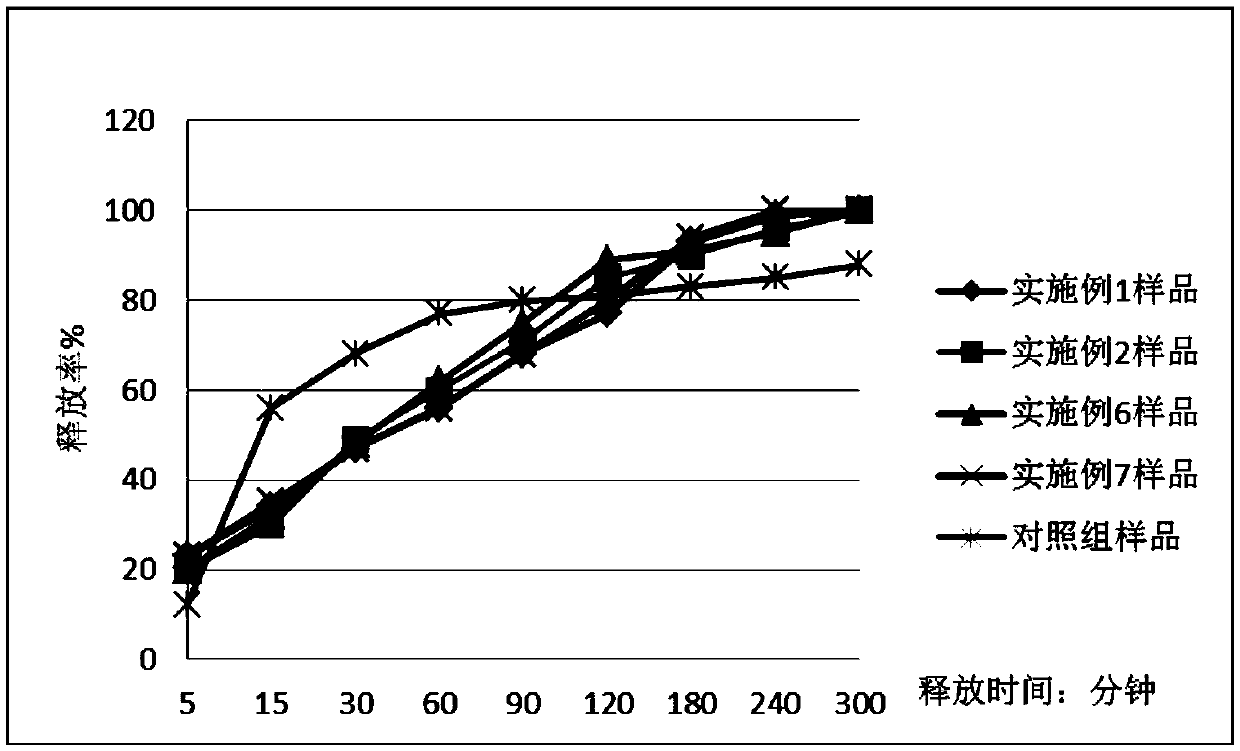
Image
Examples
Embodiment 1
[0045]
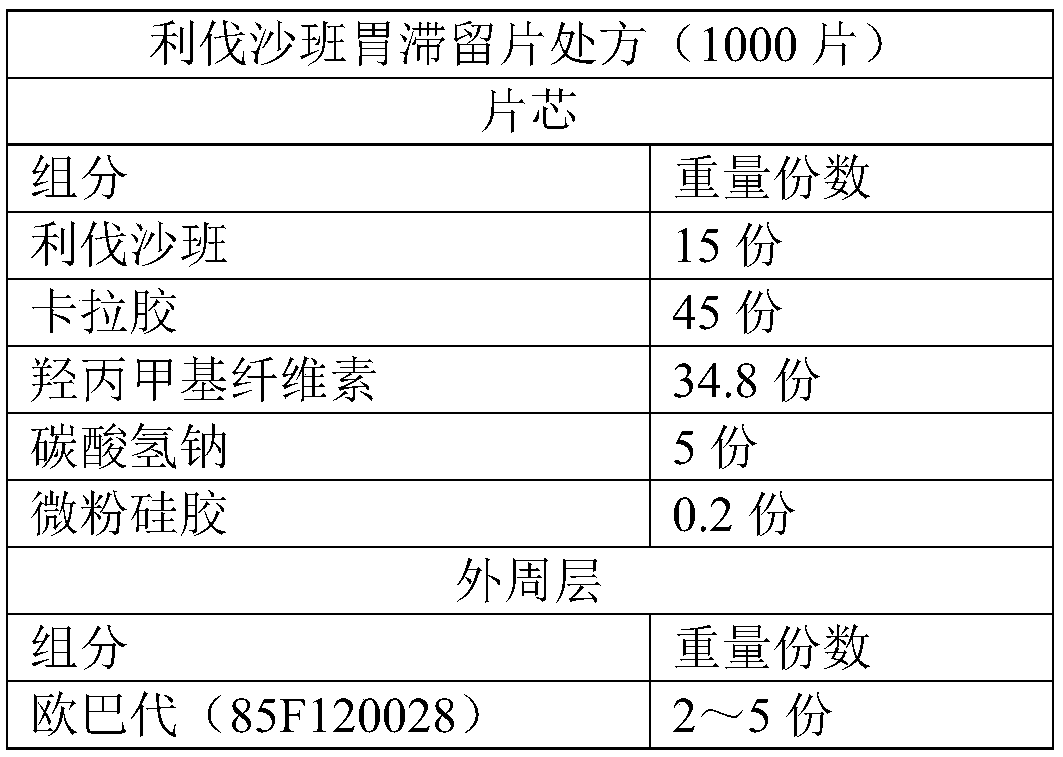
[0046]The preparation method of rivaroxaban gastric retention tablet comprises the steps:
[0047] 1) Mix 15 parts of micronized rifaxaban, 22.5 parts of carrageenan (50% by weight) and 34.8 parts of hydroxypropylmethylcellulose with an appropriate amount of water, extrude and granulate, and then granulate at 50°C Bake for 2 hours to obtain drug-containing granules;
[0048] 2) Dry mix the above-mentioned drug-containing granules, 22.5 parts of carrageenan, 5 parts of sodium bicarbonate and 0.2 part of micropowder silica gel evenly, and then use a high-speed tablet press to compress tablets to obtain tablet cores. The hardness of the tablet cores is controlled by the tablet press to 50~70N;
[0049] 3) Take Opadry (85F120028) and add purified water, stir and dissolve to prepare a gastric-soluble coating solution with a mass percentage of 10%, and use a high-efficiency coating machine to evenly spray the gastric-soluble coating solution on the tablet core until Th...
Embodiment 2
[0051]
[0052] The preparation method of the rivaroxaban gastric retention tablet in Example 2 is the same as that in Example 1, except that the prescription of the Rivaroxaban gastric retention tablet is the prescription in Example 2.
Embodiment 3
[0054]
[0055] The preparation method of the rivaroxaban gastric retention tablet in Example 3 is the same as that in Example 1, except that the prescription of the Rivaroxaban gastric retention tablet is the prescription in Example 3.
PUM
| Property | Measurement | Unit |
|---|---|---|
| hardness | aaaaa | aaaaa |
| hardness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com