Polycarboxylic acid organic ligand based on NHPI functionalization and synthesis method of ligand
A technology of organic ligands and polycarboxylic acids, applied in chemical instruments and methods, organic compound/hydride/coordination complex catalysts, organic chemistry, etc., can solve NHPI leakage, difficult to achieve catalytic effect, and poor reusability problems such as improved product purity, excellent reusability, and good molecular oxygen oxidation performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
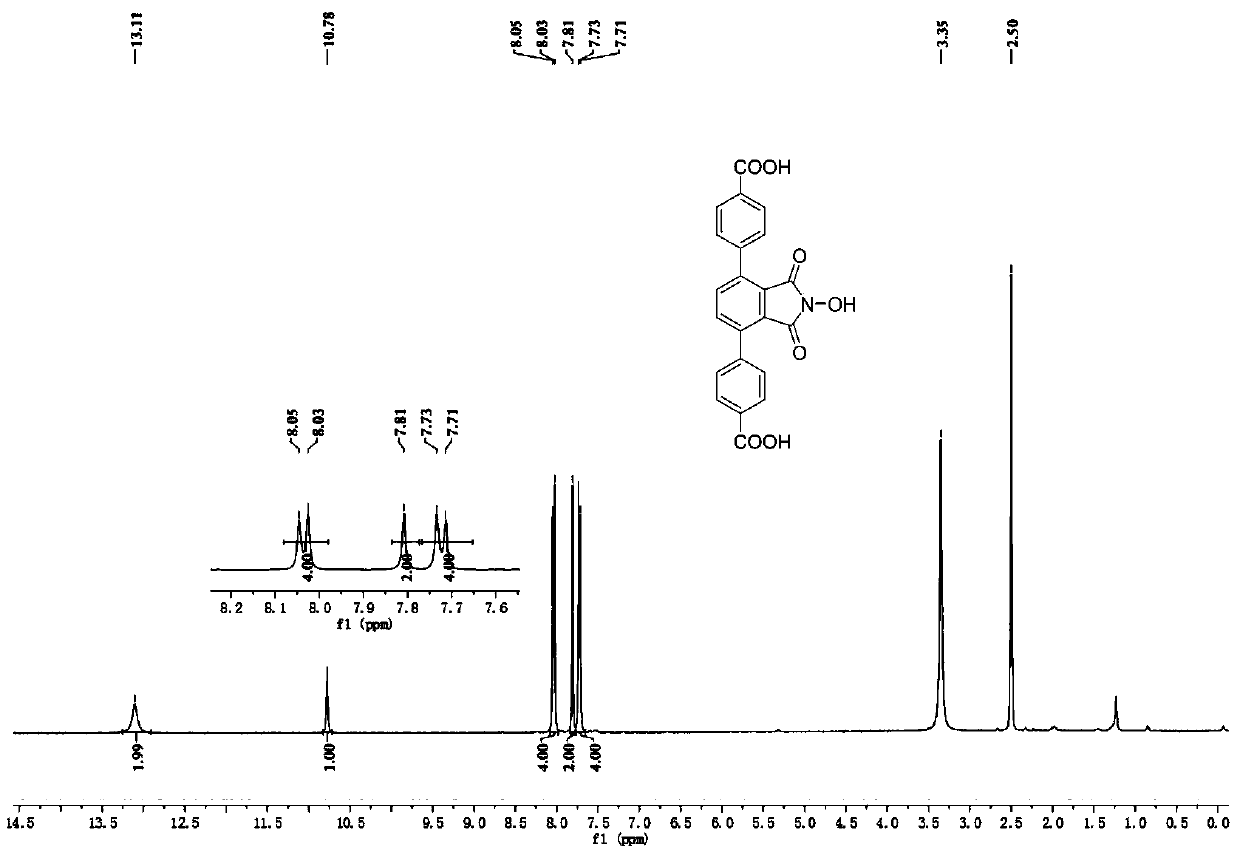
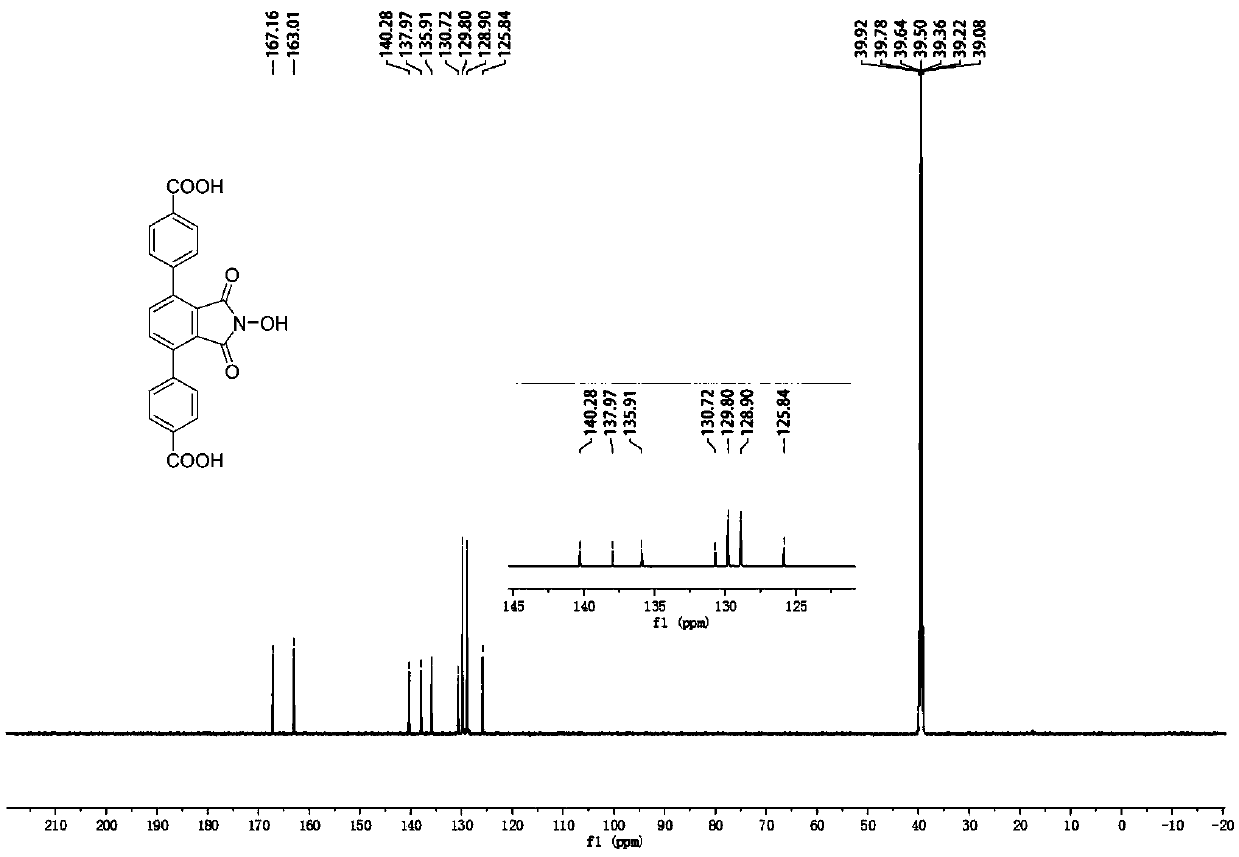
[0033] N-hydroxyphthalimide (NHPI)-modified polycarboxylic acid organic ligand (a): 4,4'-(2-hydroxy-1,3-dioxoisoindoline-4, 7-diyl) dibenzoic acid (referred to as H 2 L N-OH ) The specific structure is as follows:
[0034]
[0035] h 2 L N-OH The reaction formula of the synthetic method is as follows:
[0036]
[0037] Preparation of polycarboxylic acid organic ligands (a) based on NHPI functionalization: in N 2Add 6.3mmol of 1,4-dibromo-2,3-dimethylbenzene, 14.1mmol of 4-methylphenylboronic acid, 25.6mmol of sodium carbonate, 0.2mmol of tetraphenylphosphopalladium, and 30ml of toluene to a 100ml three-necked flask under protection. , 6ml of deionized water, and then replaced with nitrogen three times, and then heated to 90°C with an oil bath under magnetic stirring, and reacted for 6h. After the reaction, wash with water, extract, dry and remove the organic solvent under reduced pressure. The product is separated by column chromatography (petroleum ether) with a y...
Embodiment 2
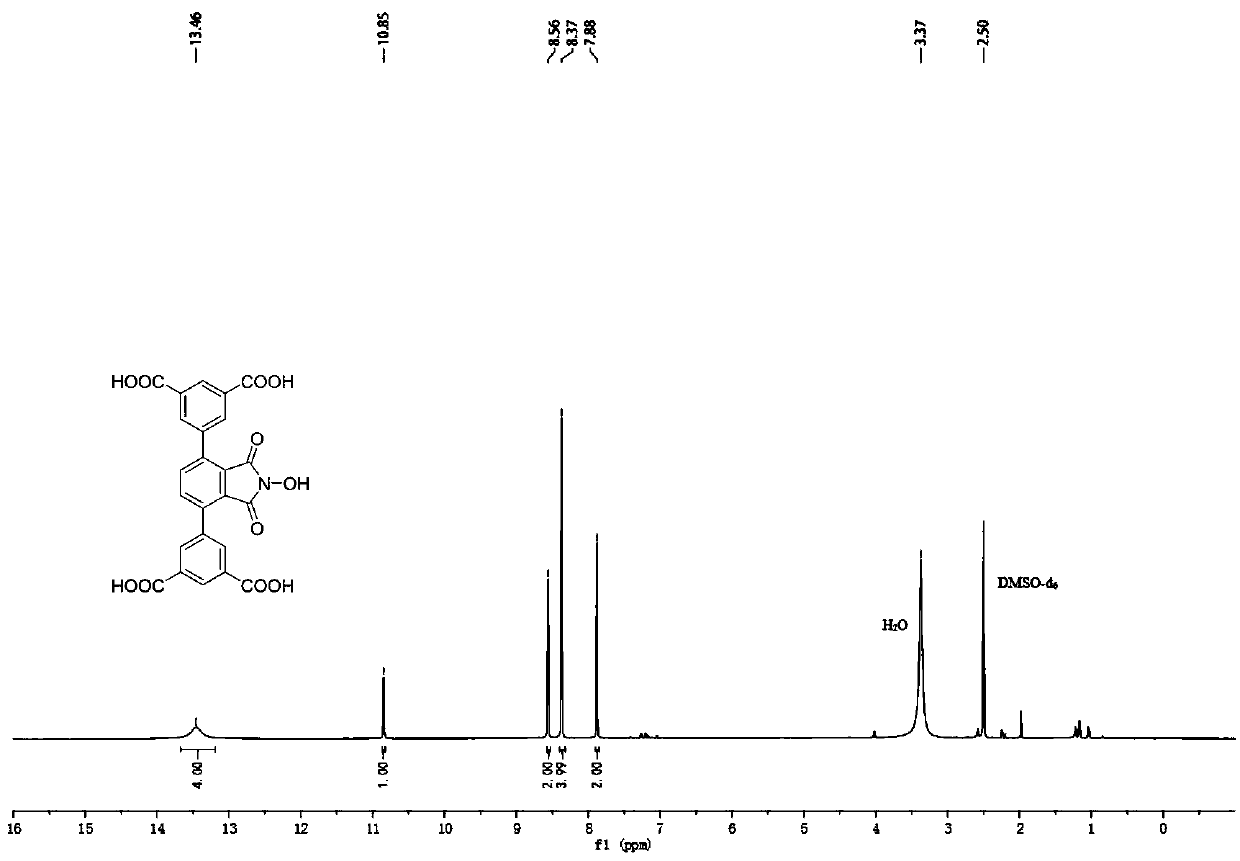
[0043] N-hydroxyphthalimide (NHPI) modified polycarboxylic acid organic ligand (b): 5,5'-(2-hydroxy-1,3-dioxoisoindoline-4,7 -diyl) diisophthalic acid (referred to as H 4 L N-OH ) The specific structure is as follows:
[0044]
[0045] h 4 L N-OH The reaction formula of the synthetic method is as follows:
[0046]
[0047] Preparation of polycarboxylic acid organic ligands (b) based on NHPI functionalization: in N 2 Add 6.3mmol of 1,4-dibromo-2,3-dimethylbenzene, 13.5mmol of 3,5-dimethylphenylboronic acid, 25.6mmol of potassium carbonate, and 0.2mmol of tetrakistriphenylphosphopalladium to a 100ml three-necked flask under protection. , 30ml of 1,4-dioxane, 6ml of deionized water, and replaced with nitrogen three times, then heated to 90°C with an oil bath under magnetic stirring, and reacted for 6h. After the reaction, wash with water, extract, dry and remove the organic solvent under reduced pressure. The product is separated by column chromatography (petroleum et...
Embodiment 3
[0053] Weigh the ligand (a), ZrCl according to the molar ratio of 1:1.2:25 4 , Benzoic acid in a glass bottle, then add N,N'-dimethylformamide (DMF) solution, ultrasonic for 15 minutes to clarify the solution, and then place the solution in a constant temperature oven at 85°C for 3 days to obtain a white powder Precipitation, the obtained white powder precipitate was washed with DMF, soaked in acetone for 2 days, and activated in vacuum at 100° C. for 8 hours. The crystal yield calculated based on the ligand was about 65%. The PXRD test results show that the obtained white powder has good crystallinity. The SEM scanning results show that the obtained white powder has a regular octahedral structure with uniform size. The BET test results show that the specific surface area of the obtained material is 2557.8386m 2 .g -1 , thus proving that the target metal organic framework material MOFs (UiO-68-nhpi) was successfully prepared. Then using the obtained MOFs (UiO-68-nhpi) as a...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com