Multi-copper oxidase, coding gene thereof, recombinant vector, recombinant strain and application
A technology for multi-copper oxidase and copper oxidase activity, applied in the field of enzyme engineering, can solve the problems of high degradation rate of aflatoxin, no measurement of actual degradation, and high cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0054] Embodiment 1: cDNA synthesis and cloning of multiple copper oxidase gene
[0055] The strain was derived from the Stenotrophomonas CW117 obtained in the laboratory in the early stage. Through gene sequence determination, the open reading frame nucleotide sequence of the multi-copper oxidase gene was analyzed, and the upstream primer of the primer that amplified the complete coding reading frame was designed ( LA-F) (SEQ ID NO.3): 5'-ATCTGGTTCCGCGT GGATCC ATGGCCGCCGCGTTGC-3'; downstream primer (LA-R) (SEQ ID NO.4): 5'-TCACGATGCGGCCG CTCGAG TCACCGCGCCATCCACAC-3', respectively introduce restriction endonuclease sites on the upstream and downstream primers (depending on the expression vector selected, BamHI and XhoI enzyme cutting sites have been added in the present invention, which have been underlined, The sequence in italics is the homologous sequence at the downstream end of the pGEX-4T-1 vector), and the polycopper oxidase gene encoding the amino acid shown in SEQ ...
Embodiment 2
[0056] Example 2: Heterologous Expression of Stenotrophomonas Multicopper Oxidase Gene
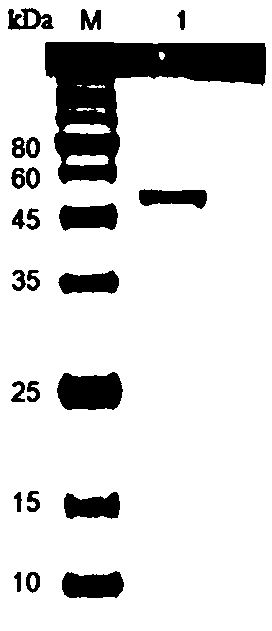
[0057] The E. coli BL21(DE3) / pGEX-4T-1 / Lac1 transformant obtained in Example 1 was cultured overnight on a shaker in 100 mL LB liquid medium containing 100 μg / mL ampicillin. Draw 1.0 mL of seed bacteria liquid and inoculate it into fresh 100 mL of LB liquid medium (containing 100 μg / mL ampicillin), and culture at 37° C. with shaking at 180 r / min. When the bacteria solution OD 600 When it reaches 0.6-0.8, add 0.2mmol / mL IPTG (isopropylthiogalactoside) to induce expression at 16°C for 4h. Refrigerate and centrifuge at 7000r / min for 10min, discard the supernatant. Suspend the cells with 10 mL of 1×phosphate buffered saline (PBS), and disrupt the cells by ultrasonic in an ice bath. The supernatant was collected by centrifugation, and after the supernatant was filtered, the expressed recombinant enzyme was purified by GST affinity chromatography to obtain pure enzyme. SDS-PAGE electrophores...
Embodiment 3
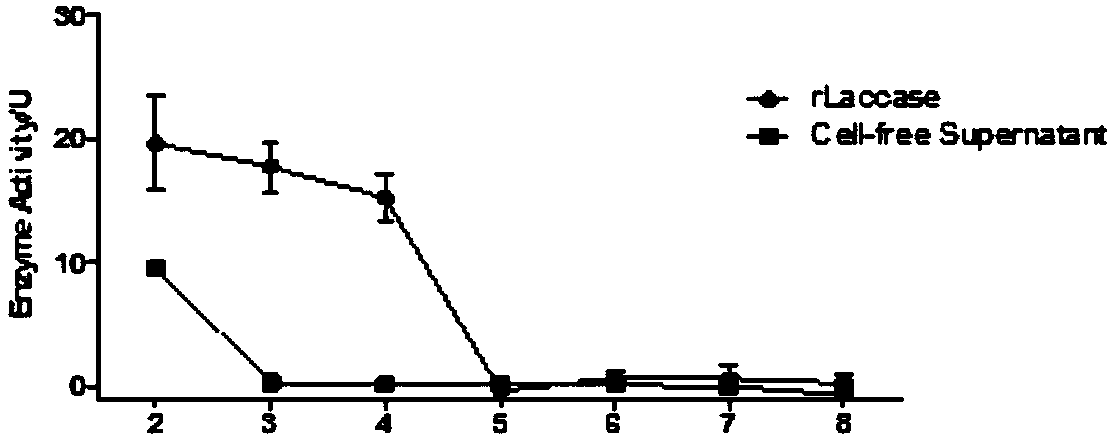
[0058] Example 3: Application of heterologous recombinant multi-copper oxidase in the degradation of aflatoxin AFB1
[0059] 1. Experimental materials
[0060] The enzyme preparation is a recombinant protein, and other reagents are analytically pure chemical reagents.
[0061] 2. Experimental method
[0062] Take 250 μL (1.0 mg / mL) of the purified recombinant multi-copper oxidase in Example 2 and mix it with an equal volume of 80 μg / L aflatoxin AFB1 (diluted working solution in PBS), incubate at 35°C for 24h, and incubate at 100°C in a water bath. The reaction was terminated by boiling for 10 min. Take 600 μL of the reacted samples and mix them with 900 μL of chromatographic grade acetonitrile, and remove the denatured protein by high-speed centrifugation and 0.22 μm membrane filtration, and then detect the remaining AFB1 concentration in the samples by UPLC. The blank control group was added with an equal amount of buffer for dissolving oxidase. It was found that the degr...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Theoretical molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com