Li-Ni-Co-Mn-V-O quaternary lithium ion battery positive electrode material and preparation method thereof
A technology for lithium-ion batteries and positive electrode materials, applied in battery electrodes, secondary batteries, circuits, etc., can solve the problems of complex synthesis conditions, difficult control, poor cycle and rate performance, etc., achieve simple synthesis process and improve material stability , Good product stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
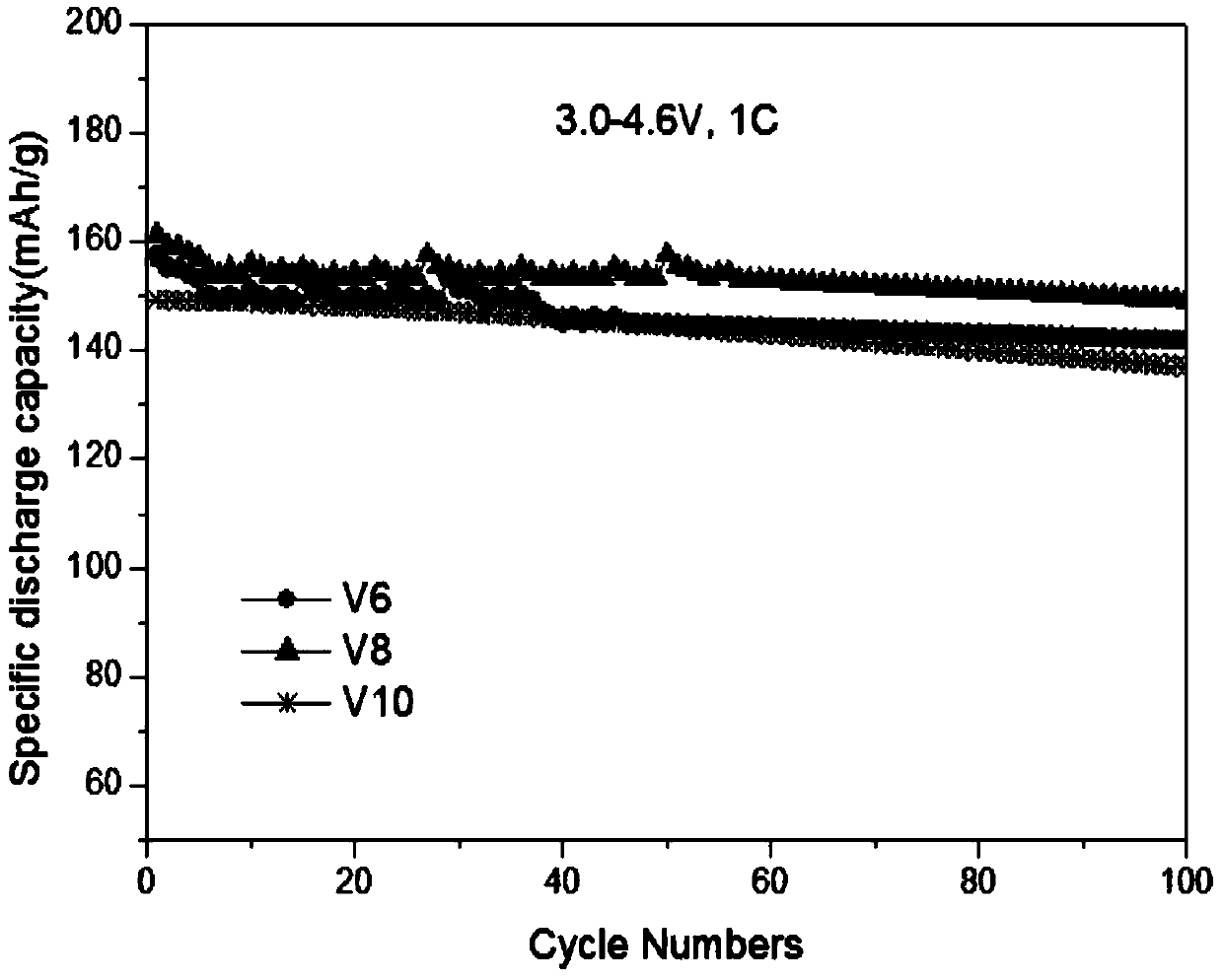
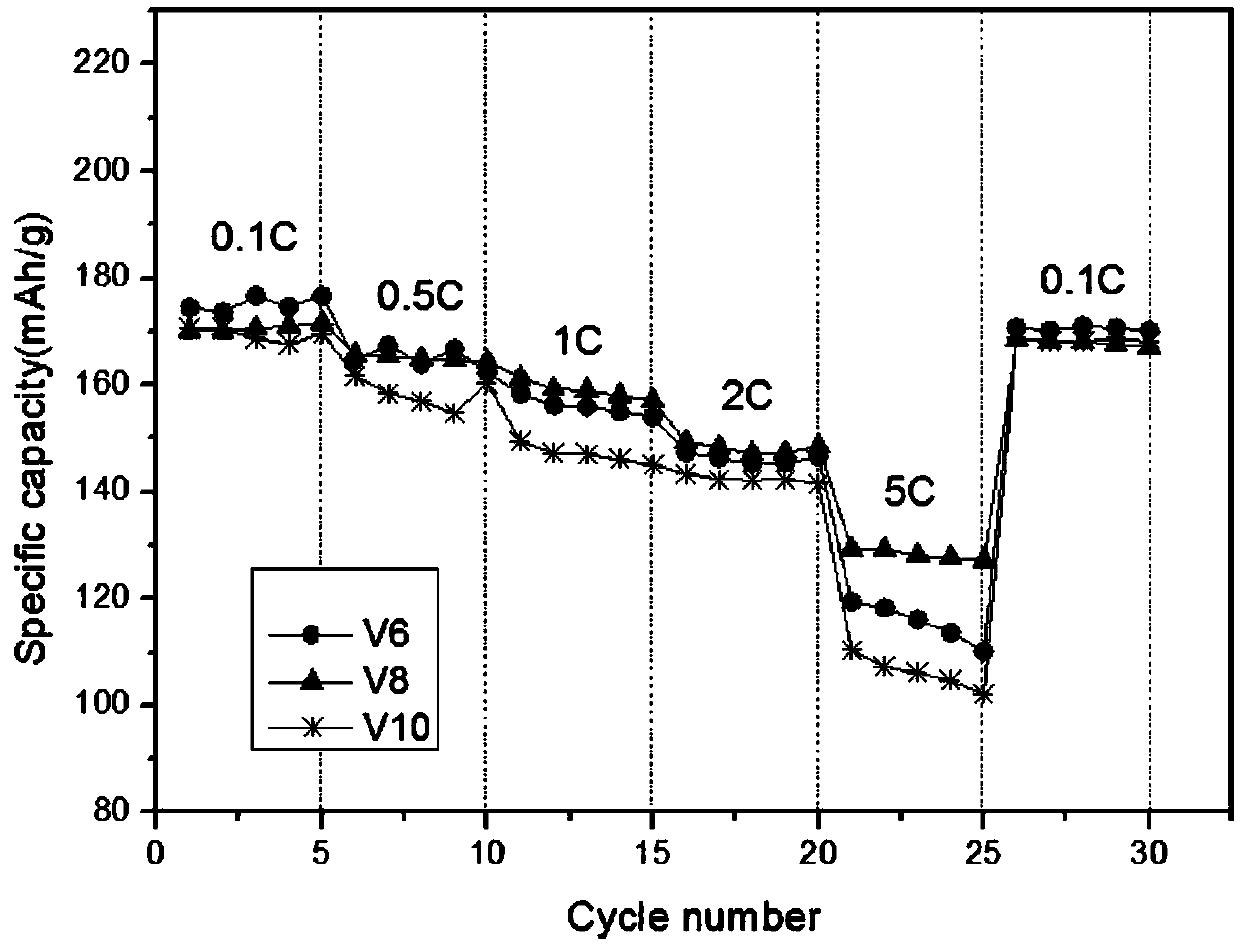
[0020] (1) First calculate and weigh 7.688g nickel acetate, 1.795g cobalt acetate, 2.520g manganese acetate according to the molar ratio of each element as Ni:Co:Mn:V=0.6:0.14:0.2:0.06 (V6), and put them together Add it into deionized water to fully dissolve at 50°C, then add 0.362g of ammonium metavanadate to the above solution for dissolution, and finally make it to 100ml to prepare solution A;
[0021] (2) Add 7.459g of oxalic acid (15% excess) into deionized water to fully dissolve, and finally adjust the volume to 100ml to prepare solution B;
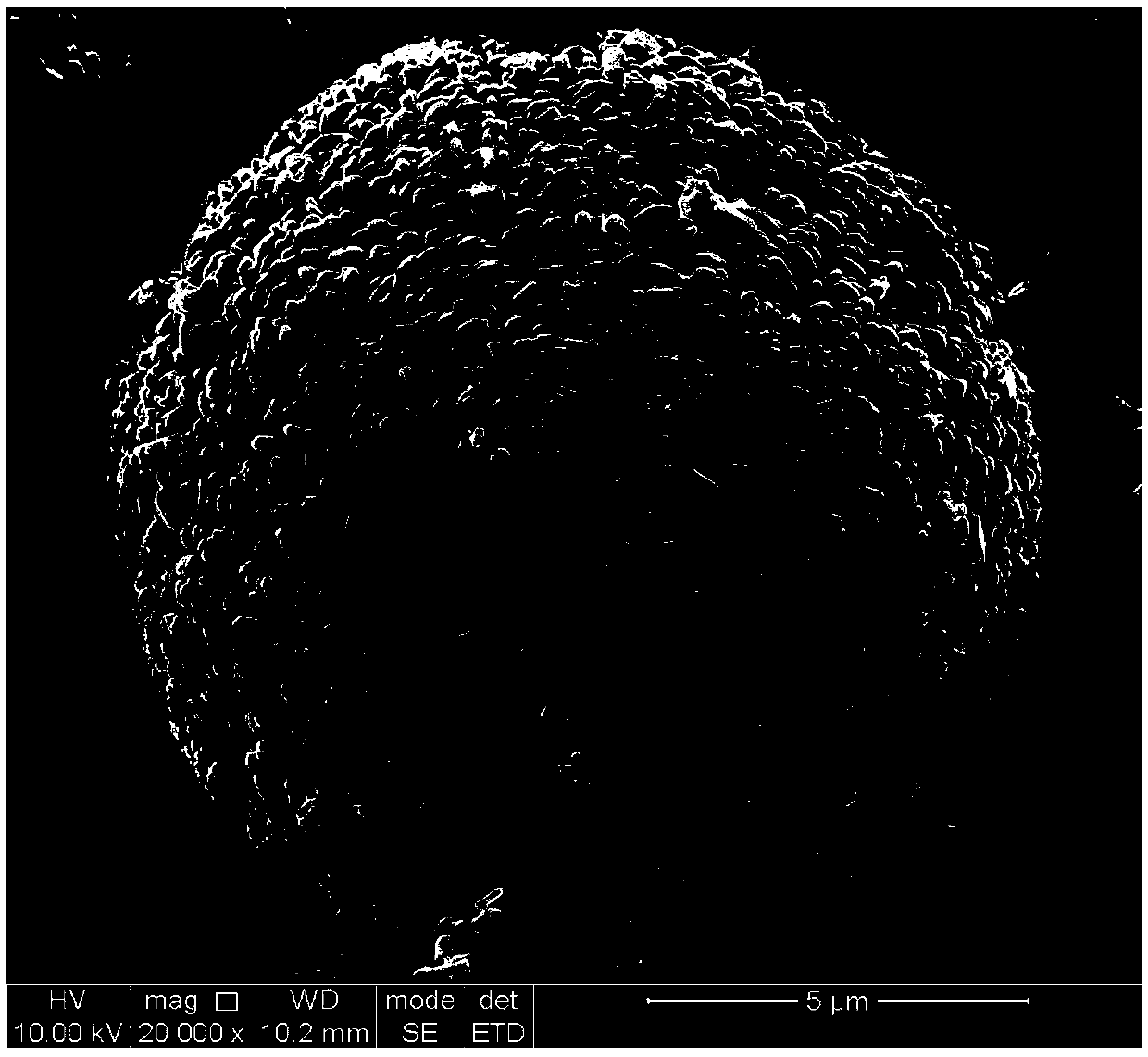
[0022] (3) Using solution B as the bottom liquid, add solution A to solution B at a rate of 6mL / min for stirring and mixing, the stirring rate is 700rpm, the temperature is 50°C, the pH is 6.5 (controlled by ammonia water), and the reaction time is 8h; After the end, the precipitate was suction filtered, washed, and dried to obtain a uniform co-precipitated spherical nickel-cobalt-manganese oxalate precursor. The drying temperature...
Embodiment 2
[0026] (1) First calculate and weigh 7.688g nickel acetate, 1.539g cobalt acetate, 2.524g manganese acetate according to the molar ratio of each element as Ni:Co:Mn:V=0.6:0.12:0.2:0.08 (V8), and put them together Add it into deionized water at 50°C to fully dissolve, then add 0.482g of ammonium metavanadate to the above solution for dissolution, and finally set the volume to 100ml to prepare solution A;
[0027] (2) Add 7.459g of oxalic acid (15% excess) into deionized water to fully dissolve, and finally adjust the volume to 100ml to prepare solution B;
[0028](3) Using solution B as the bottom liquid, add solution A to solution B at a rate of 7mL / min for stirring and mixing, the stirring rate is 700rpm, the temperature is 60°C, the pH is 6.5 (controlled by ammonia water), and the reaction time is 8h; After the end, the precipitate was suction filtered, washed, and dried to obtain a uniform co-precipitated spherical nickel-cobalt-manganese oxalate precursor. The drying tempe...
Embodiment 3
[0033] (1) First calculate and weigh 7.71g of nickel acetate, 1.285g of cobalt acetate, and 2.529g of manganese acetate according to the molar ratio of each element as Ni:Co:Mn:V=0.6:0.1:0.2:0.1 (V10). Add it into deionized water at 50°C to fully dissolve, then add 0.604g of ammonium metavanadate to the above solution for dissolution, and finally make it to 100ml to prepare solution A;
[0034] (2) Add 7.285g of oxalic acid (15% excess) into deionized water to fully dissolve, and finally adjust the volume to 100ml to prepare solution B;
[0035] (3) Using solution B as the bottom liquid, add solution A to solution B at a rate of 8mL / min for stirring and mixing, the stirring rate is 700rpm, the temperature is 70°C, the pH is 6.5 (controlled by ammonia water), and the reaction time is 8h; After the end, the precipitate was suction filtered, washed, and dried to obtain a uniform co-precipitated spherical nickel-cobalt-manganese oxalate precursor. The drying temperature was 90°C, ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com