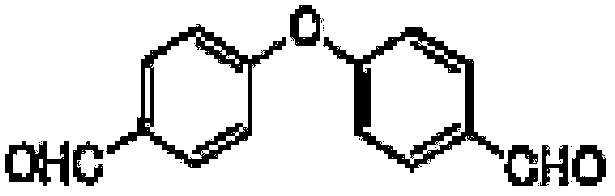
Preparation method of 4-(4-formyl phenoxy)benzaldehyde
A formylphenoxy, hydroxybenzaldehyde technology, applied in the field of preparation of 4-benzaldehyde, can solve the problems of difficulty in industrialization, high risk, low yield, etc., and achieves low price, low production cost, and easy raw materials. the effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0050] A preparation method of 4-(4-formylphenoxy)benzaldehyde:
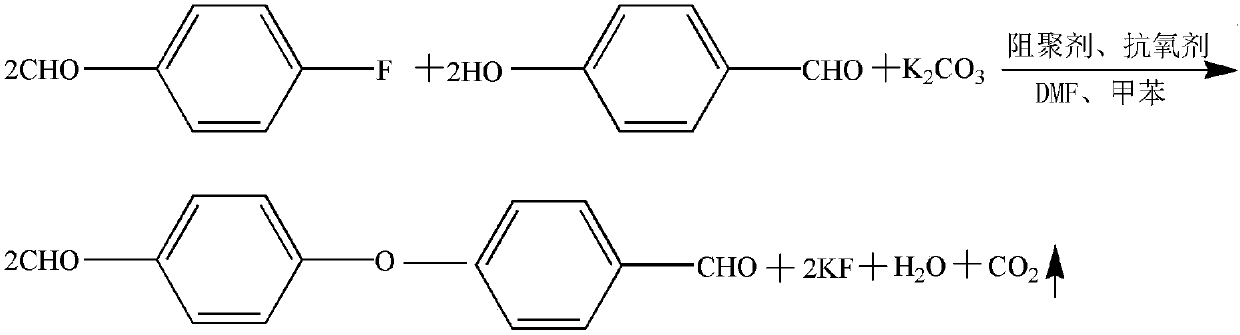
[0051] Step 1. Put 303kg of 4-hydroxybenzaldehyde, 240kg of potassium carbonate, 90.9g of phenothiazine, 121.2g of BHT, 1212kg of N,N-dimethylformamide and 242.4kg of toluene into an enamel reactor with a reflux device, stir and heat to 120 Incubate at ℃, reflux and dehydrate for 3 hours to obtain an intermediate solution;
[0052] Step 2. Add 299g 4-fluorobenzaldehyde dropwise to the intermediate solution, the dropping time is 0.5h, and the reaction is kept at 120°C for 16h, sampling HPLC detects that the 4-fluorobenzaldehyde content is less than 0.2%, the reaction is over, 4 -(4-Formylphenoxy)benzaldehyde solution;
[0053] Step: Distill toluene out under normal pressure, and stop distilling to the gas phase temperature of 145°C; reduce the temperature to 85°C, keep it warm and press filter, wash the filter cake with 200kg N,N-dimethylformamide, combine the filtrate and washing liquid, stir, Add 1212kg of hot water...
Embodiment 2
[0055] A preparation method of 4-(4-formylphenoxy)benzaldehyde:
[0056] Step 1. Put 303kg of 4-hydroxybenzaldehyde, 205.8kg of potassium carbonate, 151.5g of phenothiazine, 60.6g of BHT, 909kg of N,N-dimethylacetamide and 272.2kg of toluene into an enamel reactor with a reflux device, stir and heat to Keep warm at 130°C, reflux and dehydrate for 2 hours to obtain the intermediate solution;
[0057] Step 2. Add 304.9kg 4-fluorobenzaldehyde dropwise to the intermediate solution, the dropping time is 0.5h, and the reaction is kept at 130°C for 14h, sampling HPLC detects that the 4-fluorobenzaldehyde content is less than 0.2%, and the reaction is over. 4-(4-formylphenoxy)benzaldehyde solution;
[0058] Step: Distill toluene out under normal pressure, distill to the gas phase temperature of 145°C and stop; lower the temperature to 90°C, keep it hot and filter with pressure, wash the filter cake with 200kg N,N-dimethylformamide, combine the filtrate and washing liquid, stir, Add 1091kg ...
Embodiment 3
[0060] Step 1. Combine 303kg of 4-hydroxybenzaldehyde, 274.3kg of potassium carbonate, 33g of phenothiazine, 151.5g of BHT, 1515kg of N,N-dimethylformamide and dimethyl sulfoxide (mass ratio of 1:1) and 151.5 The mixture of kg toluene and xylene (the mass ratio is 1:1) is put into an enamel reactor with a reflux device, stirred and heated to 110°C for heat preservation, refluxed and dehydrated for 4 hours to obtain an intermediate solution;
[0061] Step 2: Add 293.3kg 4-fluorobenzaldehyde dropwise to the intermediate solution, the dropping time is 0.5h, and the reaction is kept at 110°C for 17h, sampling HPLC detects that the 4-fluorobenzaldehyde content is less than 0.2%, the reaction is over, 4-(4-formylphenoxy)benzaldehyde solution;
[0062] Step: Distill toluene out under normal pressure, distill to the gas phase temperature of 145°C and stop; lower the temperature to 80°C, keep it warm and press filter, wash the filter cake with 200kg N,N-dimethylformamide, combine the filtra...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com