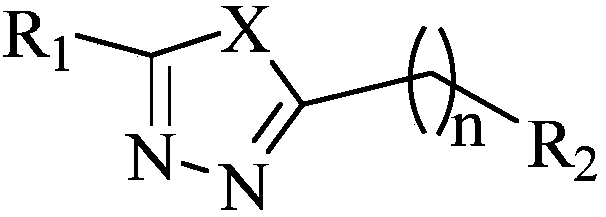
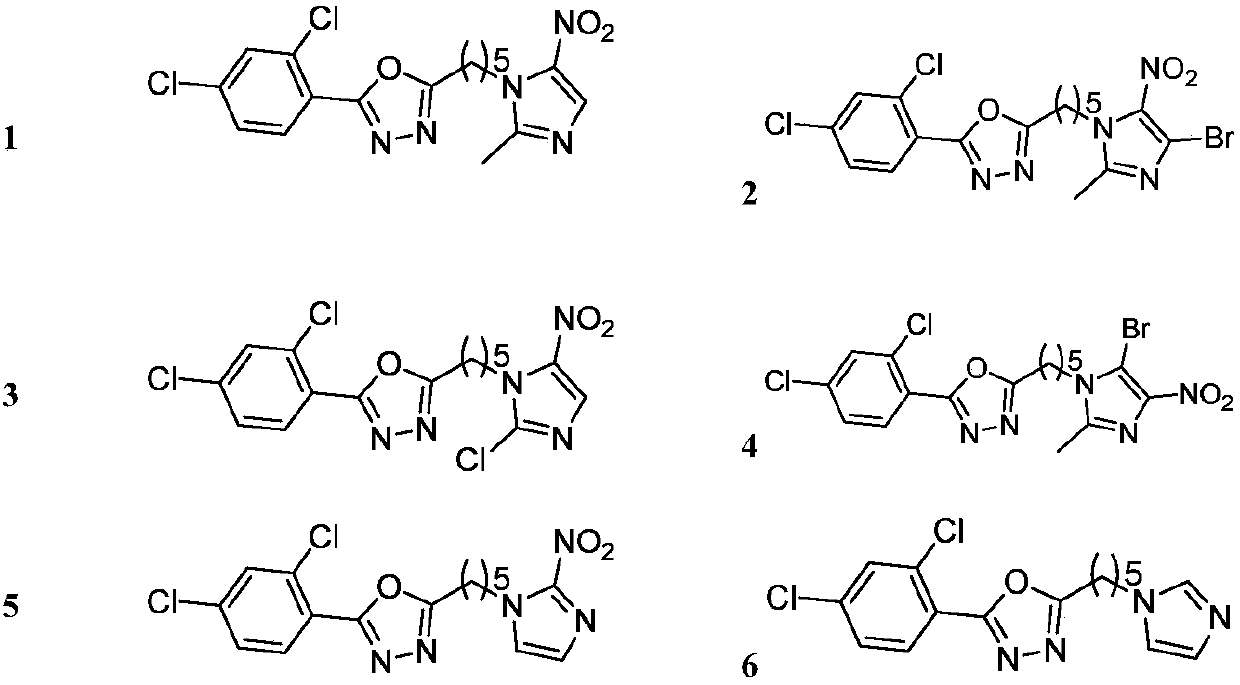
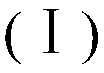Heterocycle substituted 1,3,4-oxadiazole compound and preparation method and application thereof
A kind of azole compound and compound technology, applied in the field of medicinal chemistry
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0066] Example 1: Preparation of intermediate 2-(5-bromopentyl) 5-(2,4-dichlorophenyl)-1,3,4-oxadiazole to synthesize 2,4-dichlorobenzohydrazide, Reference [Wang, P.Y; Zhou, L; Zhou, J; Wu, Z.B; Xue, W; Song, B.A; Yang, S. Synthesis and antibacterial activity of pyridinium-tailored 2,5-substituted-1,3,4 -oxadiazole thioether / sulfoxide / sulfone derivatives. Bioorg Med Chem Lett, 2016, 26, 1214-1217]. It (0.5g, 2.44mmol) was added to 8mL POCl containing 6-bromohexanoic acid (0.48g, 2.44mmol) 3 In the solution, react at 75°C for 10h. Remove POCl under reduced pressure 3 , and then add 70mL of ethyl acetate to the residue for extraction, the organic layer was washed with water, potassium carbonate aqueous solution, brine, dried with anhydrous sodium sulfate, filtered, and then precipitated. Using PE:EA=8:1 as the eluent, the intermediate was obtained as a white solid with a yield of 73.2% and a melting point of 164.3-165.6°C.
Embodiment 2
[0067] Example 2: 2-(2,4-dichlorophenyl)-5-(5-(2-methyl-5-nitro-1H-imidazol-1-yl)pentyl)-1,3,4 - Preparation of oxadiazole
[0068] 2-(5-bromopentyl)-5-(2,4-dichlorophenyl)-1,3,4-oxadiazole 4 (0.2g, 0.55mmol), 2-methyl-5-nitro -1H-imidazole (0.14g, 1.10mmol) and K 2 CO 3 Dissolve in DMF (7 mL), and stir at 60° C. for 12 hours. The solvent was removed under reduced pressure and extracted with ethyl acetate. The organic layer was washed with water, brine, dried over anhydrous sodium sulfate, filtered, and stripped. Use CH 2 Cl 2 :CH 3OH=40:1 was used as the eluent to obtain the desired product as a brown solid, yield: 99.9%, melting point: 49.8-50.1°C.
[0069] The other compounds were prepared in analogy to the synthetic method steps of Examples 1 and 2 using the corresponding starting materials.
[0070] The structure, H-NMR and C-NMR data of the synthesized 1,3,4-oxadiazolylimidazole (partial heterocycle) compounds are shown in Table 1, and their physical and chemica...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com