Fluorescent probe molecule for detecting diaphorase based on benzopyranidonitrile, preparation and application thereof
A technology of benzopyranonitrile and diaphorase, applied in fluorescence/phosphorescence, material analysis through optical means, material analysis, etc., can solve the problem of rare diaphorase and achieve good stability of physical and chemical properties , easy to synthesize, stable optical properties of fluorophores
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
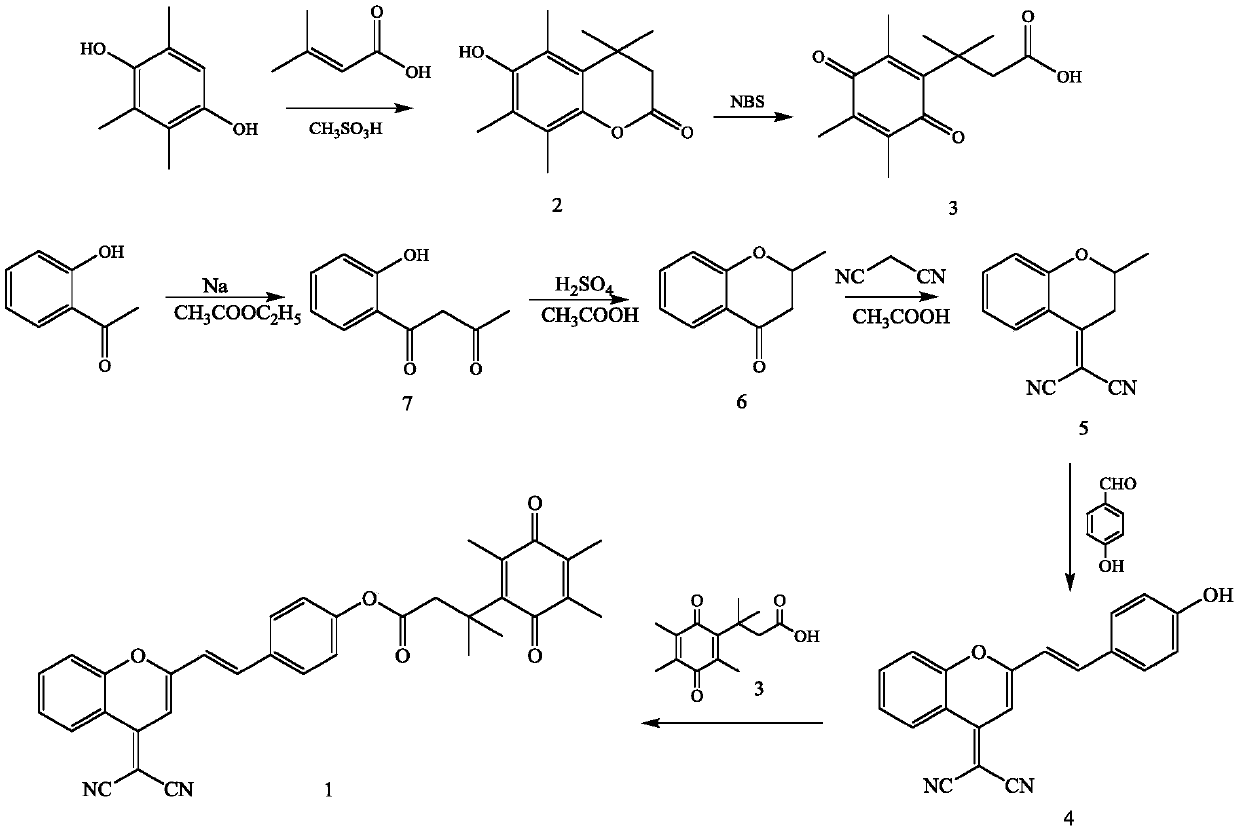
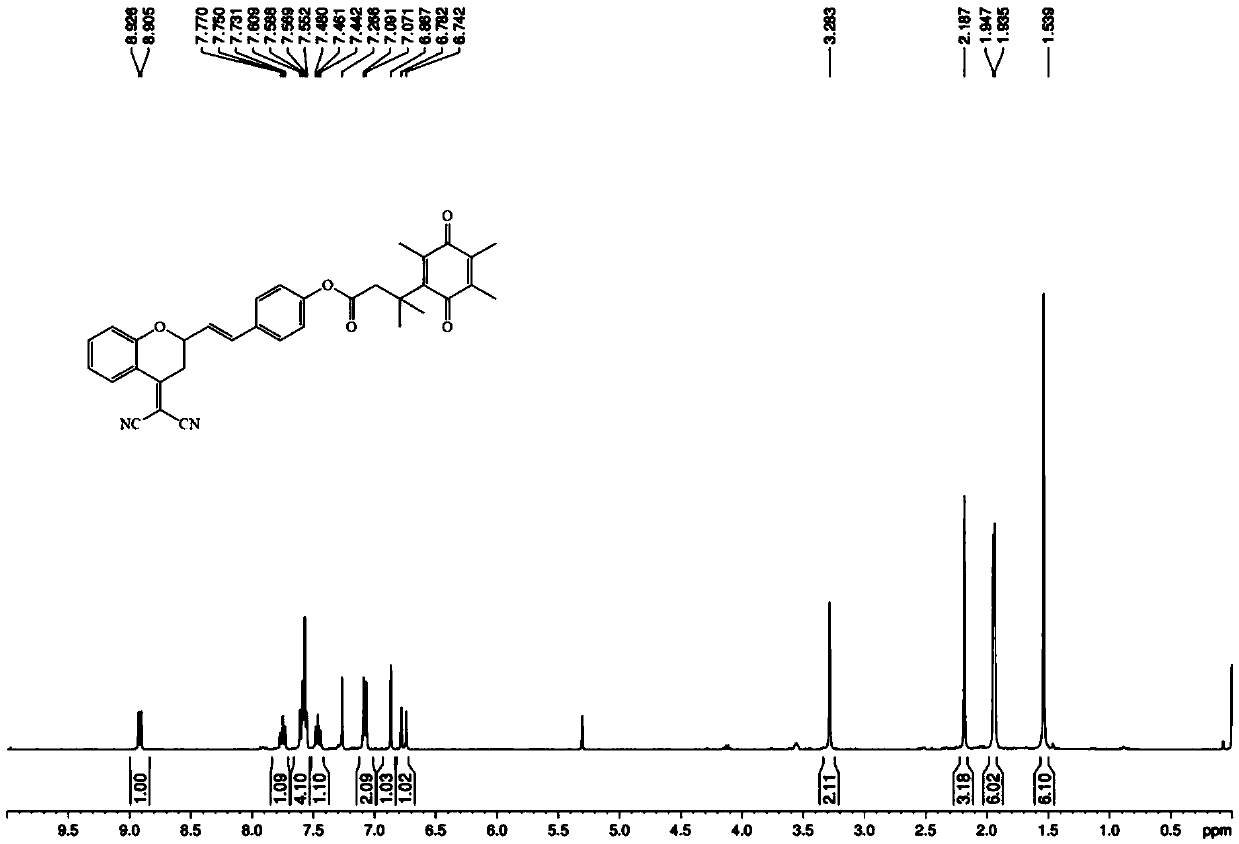
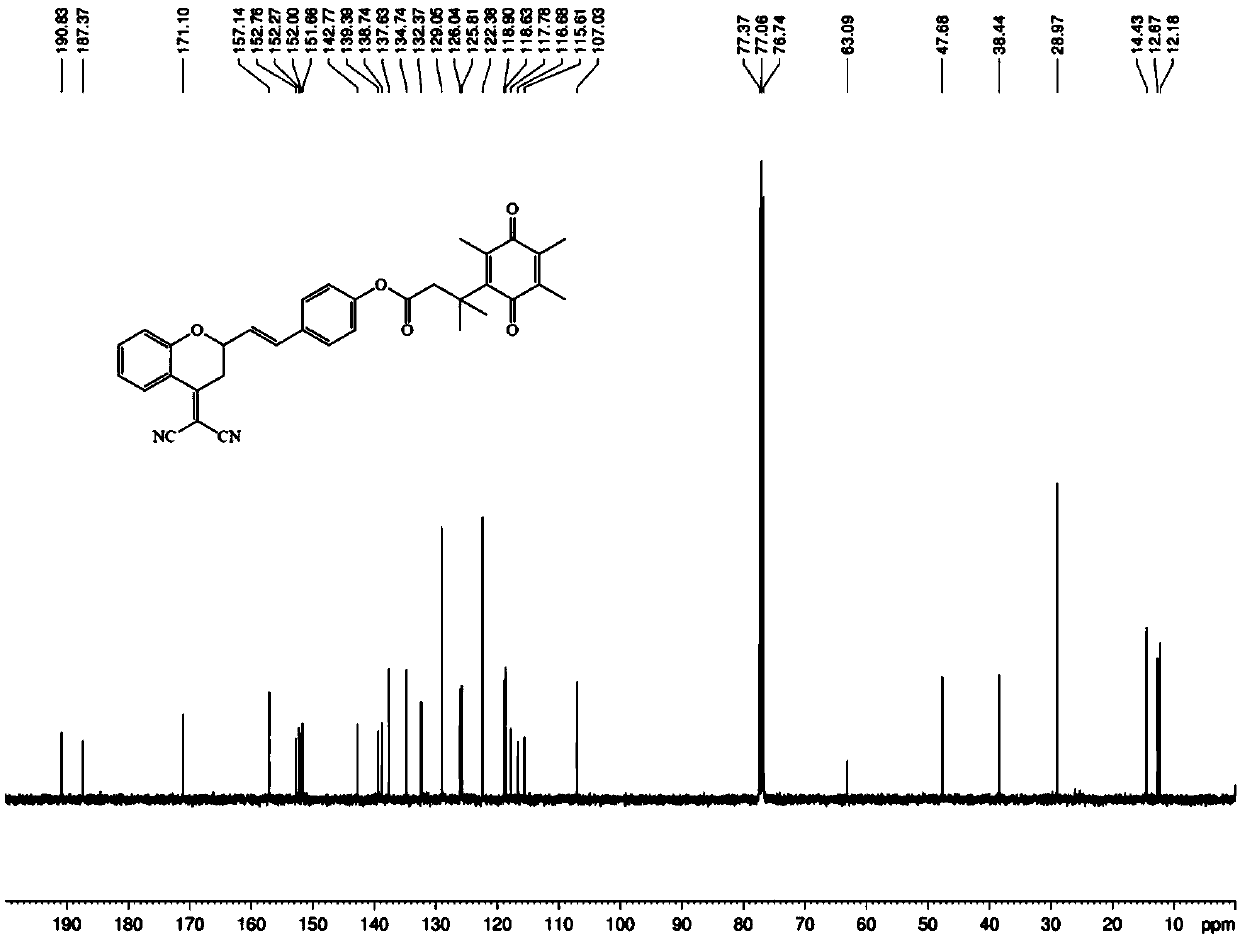
[0043] Embodiment 1: the synthesis of fluorescent probe molecule of the present invention
[0044] For specific synthetic routes, see figure 1 .
[0045] (1) Preparation of Compound 7: Dissolve 1-(2-hydroxyphenyl)ethanone (10.0g, 73.5mmol), sodium (8.00g, 0.34mmol) in 250mL ethyl acetate, react at room temperature for 8 hours, and react Suction filtration is performed after the end, the filter cake is washed twice with ethyl acetate, dissolved in deionized water, the pH of the solution is adjusted to neutrality with concentrated hydrochloric acid, extracted three times with ethyl acetate, the organic phase is retained, and dried with anhydrous sodium sulfate , the pale yellow solid obtained by distillation under reduced pressure after filtration was compound 7, and the yield was 60%.
[0046](2) Preparation of Compound 6: Dissolve Compound 7 (6.95 g, 38.9 mmol) in 70 mL of glacial acetic acid, slowly add 4.6 mL of concentrated sulfuric acid dropwise under stirring at room t...
Embodiment 2
[0053] Example 2: Selectivity of diaphorase detection fluorescent probe molecules based on benzopyranonitrile to diaphorase fluorescence detection
[0054] DMSO (dimethyl sulfoxide): PBS (0.01mol / L, pH=7.4) = 1:99 (v:v) solution was used to control the experimental conditions.
[0055] The fluorescent probe molecules of the present invention are dissolved in a solvent of DMSO:PBS=1:99 (v:v) and settled in a 100mL volumetric flask, and the concentration of the fluorescent probe molecules is prepared to be 5×10 -6 mol / L solution.
[0056] Divide the sample vials into 14 groups, add 3mL to each group of sample vials with a concentration of 5×10 -6 mol / L DMSO:PBS (0.01mol / L, pH=7.4)=1:99 (v:v) solution of the fluorescent probe molecule of the present invention, the first bottle of solution is used as a blank group, and the other 13 groups are respectively added with a concentration of 5 μL Ala, Arg, Cys, Gln, Glu, Gly, Hcy, His, Lys, Met, Thr, Val, DTD at 25 μg / μL. After the so...
Embodiment 3
[0058] Embodiment 3: Quantitative fluorescent detection of diaphorase by fluorescent probe molecule of the present invention
[0059] DMSO (dimethyl sulfoxide): PBS (0.01mol / L, pH=7.4) = 1:99 (v:v) solution was used to control the experimental conditions.
[0060] The fluorescent probe molecules of the present invention are dissolved in a solvent of DMSO:PBS=1:99 (v:v) and settled in a 100mL volumetric flask, and the concentration of the fluorescent probe molecules is prepared to be 5×10 -6 mol / L solution.
[0061] 2 mg of diaphorase (DTD) was dissolved in 800 μL of deionized water to prepare an aqueous solution of diaphorase with a concentration of 2.5 μg / μL.
[0062] Divide the sample vials into 21 groups, add 0 μL to 210 μL of diaphorase aqueous solution with a concentration of 2.5 μg / μL in each group of sample vials, and then use a concentration of 5×10 -6 DMSO:PBS (0.01mol / L, pH=7.4)=1:99 (v:v) solution of the fluorescent probe molecule of the present invention of mol / L...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com