Trifunctional benzoxazine monomer based on resveratrol and preparation method of trifunctional benzoxazine monomer
A technology of resveratrol and benzoxazine, which is applied in the field of trifunctional benzoxazine monomer and its preparation, can solve the problem of long reaction time of biological benzoxazine, insufficient heat resistance, and insufficient thermal performance and other problems, to achieve the effect of being suitable for large-scale production, low equipment requirements and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] 2-furylamine was used as the amine source.
[0030] Add 1 g (0.0044 mol) of resveratrol, 1.276 g (0.0132 mol) of 2-furan methylamine, and 0.868 g (0.0289 mol) of paraformaldehyde into the flask, add 50 ml of toluene solution, connect a condenser tube, and stir at 110 ° C And react for 4h. The filtrate after the reaction was rotary evaporated to remove the solvent to obtain 1.8 g of benzoxazine monomer with a yield of 68%. The chemical reaction equation is as follows:
[0031]
[0032] In the present embodiment, the obtained oxazine product structure is:
[0033]
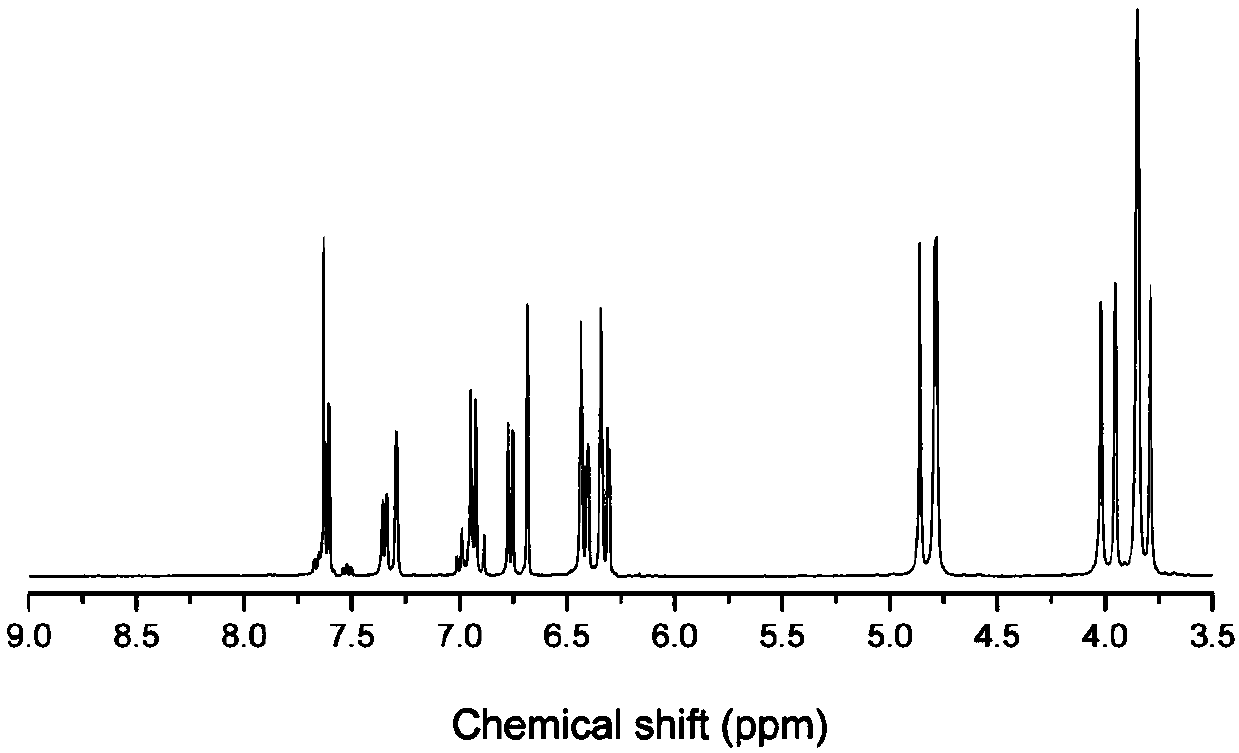
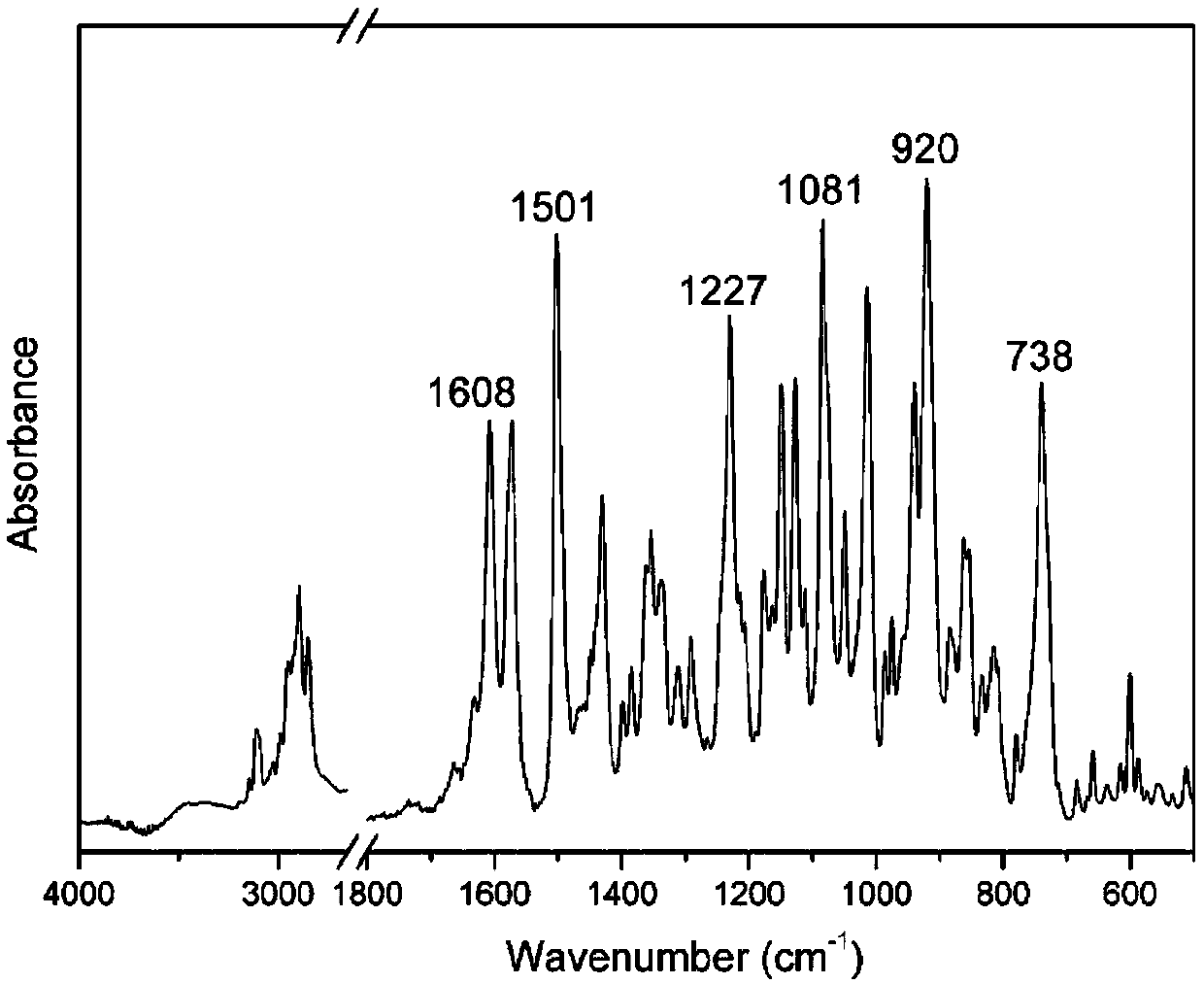
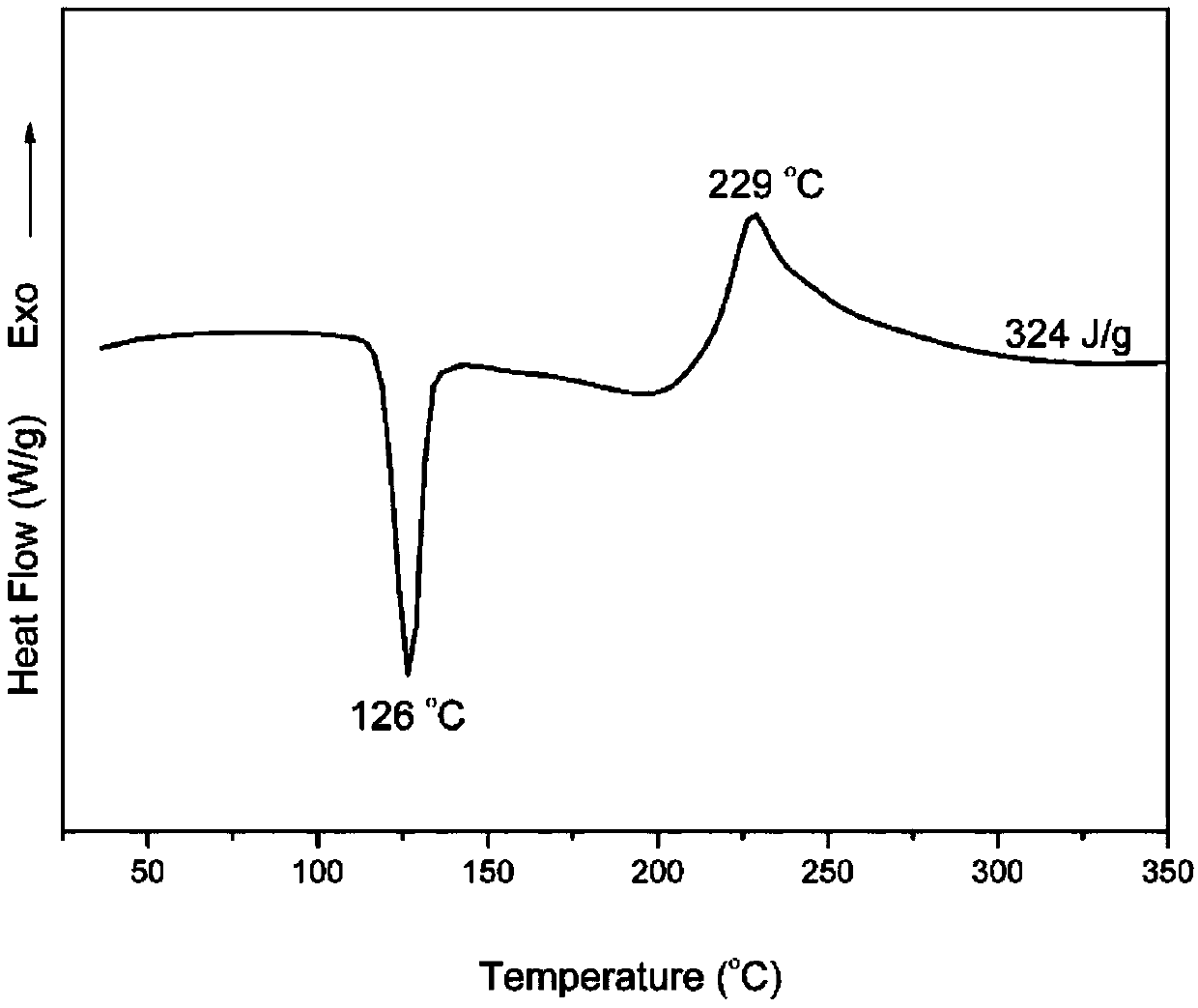
[0034] The proton nuclear magnetic resonance spectrum, Fourier transform infrared transform spectrum, DSC curve and thermogravimetric curve of this product are shown in the appendix figure 1 , attached figure 2 , attached image 3 And attached Figure 4 .
[0035] attached figure 1 Proton NMR spectrum. Chemical shifts around 4.8ppm and 3.9ppm are characteristic peaks of methylene on the oxazine...
Embodiment 2
[0037] The amine source compound 2-furylmethylamine in Example 1 was replaced by aniline. Other steps are the same as those in Example 1.
[0038] Wherein the specific chemical structural formula of aniline is: The amount of reactants was changed to: weigh 1.14g (0.005mol) of resveratrol, 1.40g (0.015mol) of aniline, and 0.99g (0.033mol) of paraformaldehyde, with a yield of 79%.
[0039]
[0040] The curing exothermic peak temperature of the trifunctional benzoxazine monomer obtained in this example is 230°C. After further curing and crosslinking, the temperature of the polybenzoxazine resin is 353°C when the thermal weight loss is 5%, and the inert gas atmosphere is 800°C. , the carbon residue rate is 61%, and the heat release energy of the flame retardant test result is 79Jg -1 K -1 .
Embodiment 3
[0042] The amine source compound 2-furylmethylamine in Example 1 was replaced by 4-chloroaniline. Other steps are the same as those in Example 1.
[0043] Wherein the concrete chemical structural formula of 4-chloroaniline is: The amount of reactants was changed to: weigh 1.14g (0.005mol) of resveratrol, 1.91g (0.015mol) of 4-chloroaniline, and 0.99g (0.033mol) of paraformaldehyde, with a yield of 75%.
[0044]
[0045] The exothermic peak temperature of the benzoxazine obtained in this example is 233°C. After further curing and crosslinking, the temperature of the polybenzoxazine resin is 357°C when the thermal weight loss is 5%, and the carbon residue is 800°C in an inert gas atmosphere The rate is 62%, and the heat release energy of the flame retardant test result is 73Jg -1 K -1 .
PUM
| Property | Measurement | Unit |
|---|---|---|
| carbon residual rate | aaaaa | aaaaa |
| carbon residual rate | aaaaa | aaaaa |
| carbon residual rate | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com