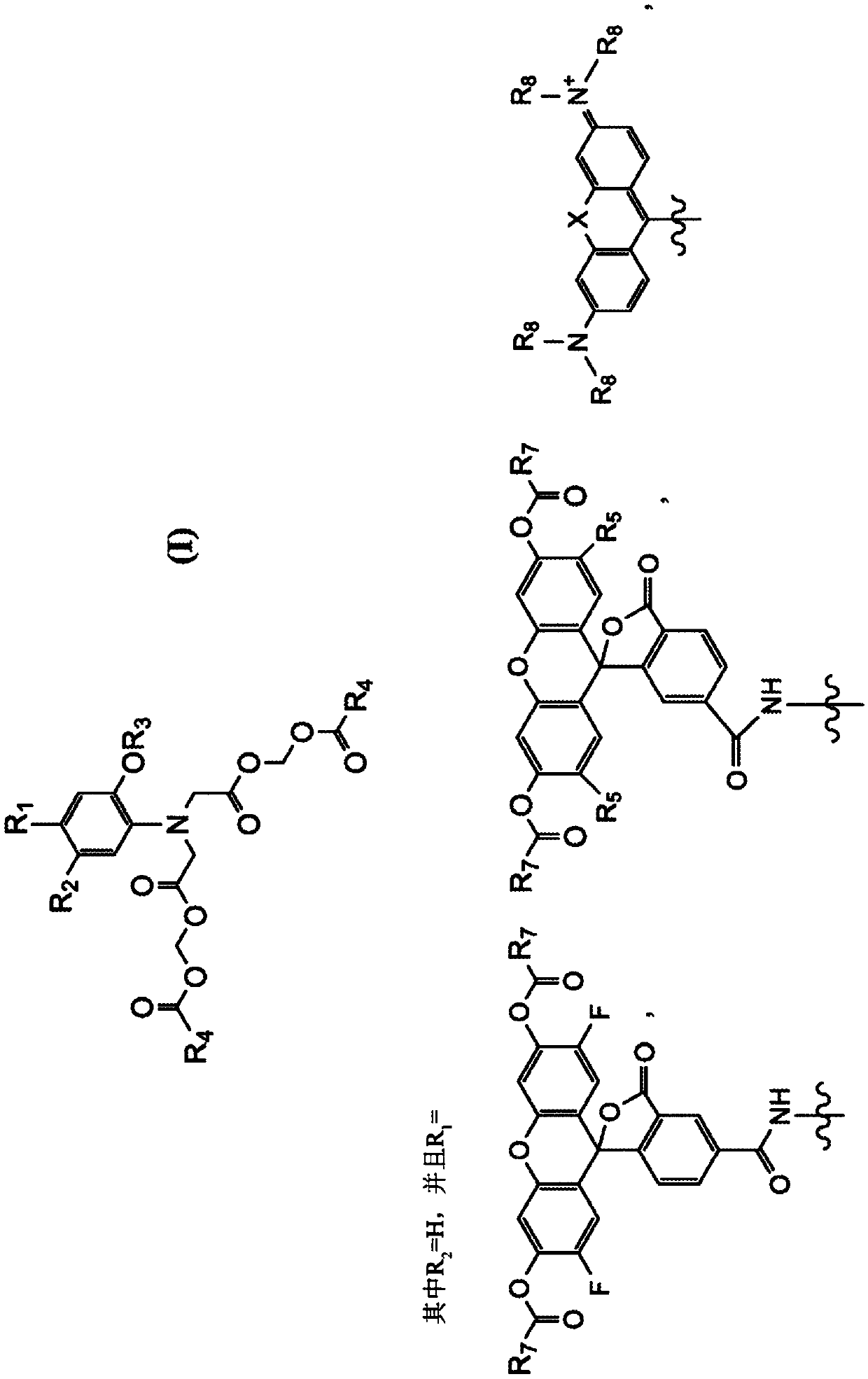
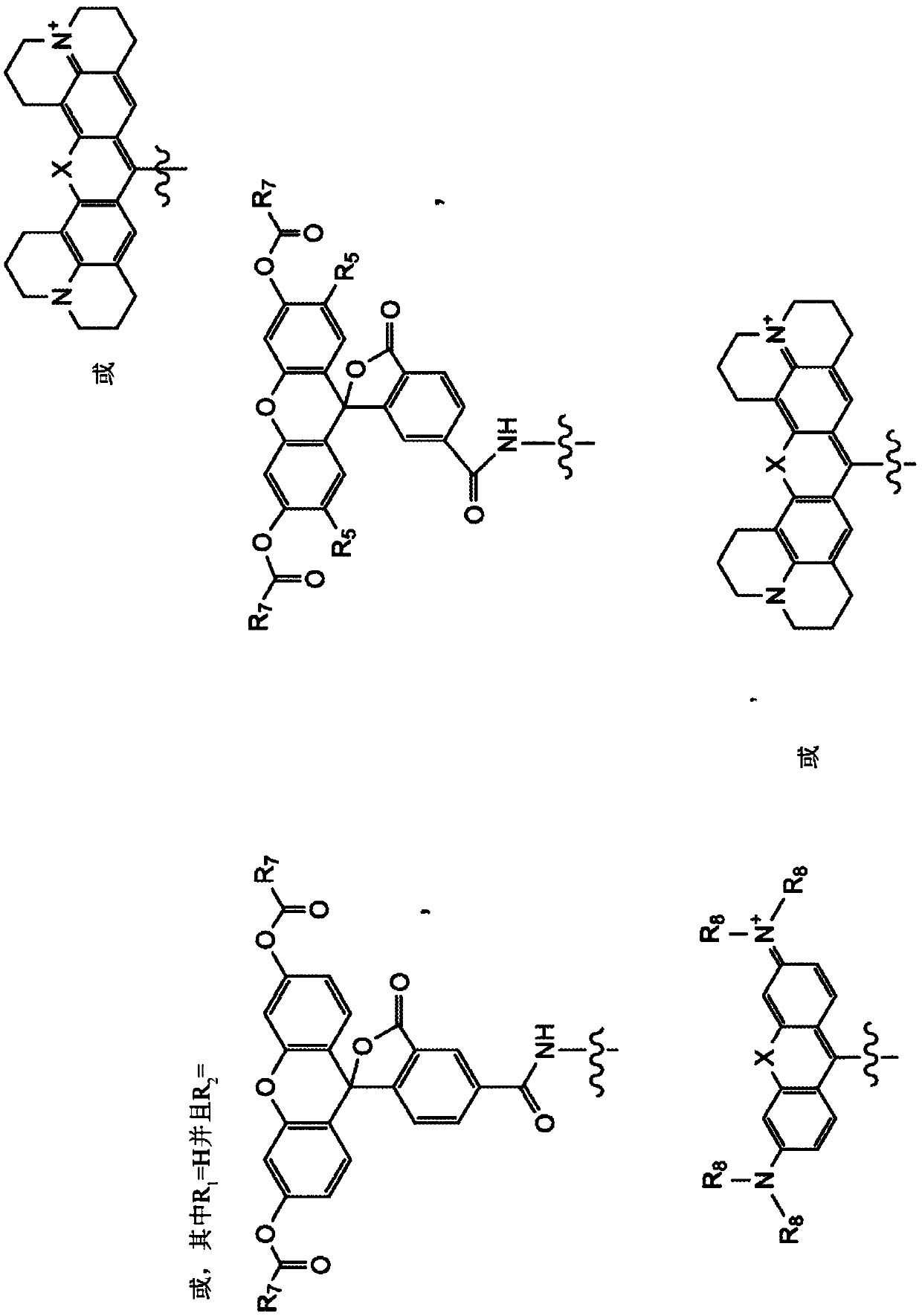
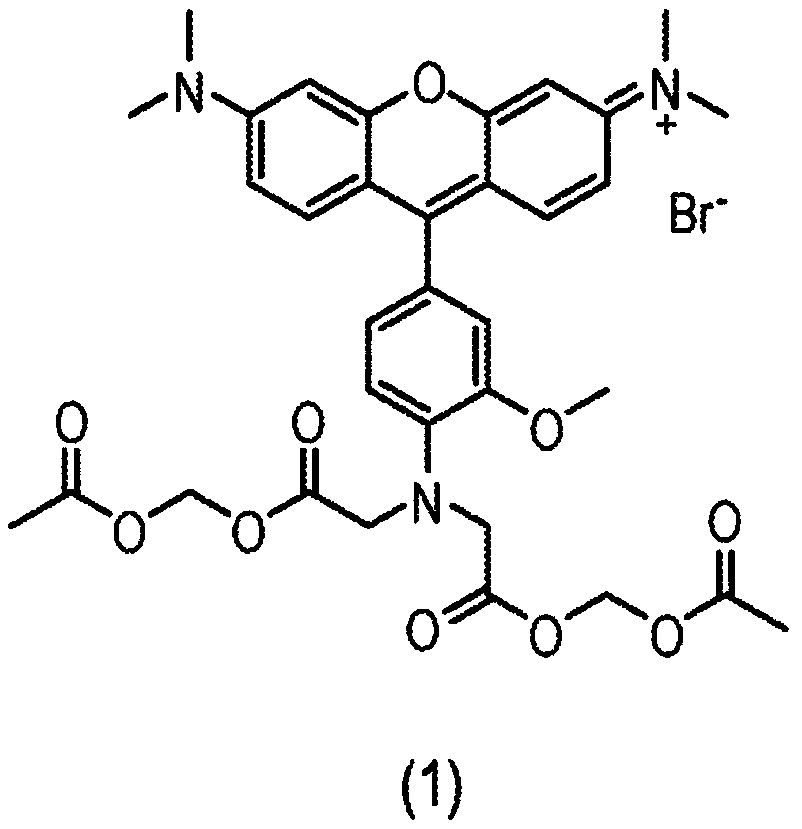
Composition and methods for measuring ion channel activity in a cell
A technology of potassium ion channels and cells, applied in the field of compositions and methods for measuring ion channel activity in cells, capable of solving problems such as insufficient sensitivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0117] Bis(acetoxymethyl)2,2'-((4-(3',6'-diacetoxy-2',7'-difluoro-3-oxo-3H-spiro[isobenzo Synthesis of furan-1,9'-xanthene]-6-formylamino)-2-methoxyphenyl)azanediyl)diacetic acid
[0118] For the synthesis of diacetate bis(acetoxymethyl) 2,2'-((4-(3',6'-diacetoxy-2',7'-difluoro-3-oxo-3H- The reaction scheme for spiro[isobenzofuran-1,9'-xanthene]-6-carboxamido)-2-methoxyphenyl)azanediyl)ester is shown in Figure 12A and Figure 12B middle. 3',6'-diacetoxy-2',7'-difluoro-3-oxo-3H-spiro[isobenzofuran-1,9'-xanthene]-6-carboxylic acid (236 mg, 0.475 mmol) was suspended in 15 mL of anhydrous DCM and the suspension was cooled in an ice-water bath. Triethylamine (66 μL, 0.47 mmol) was added to the suspension, followed by isobutyl chloroformate (73 μL, 0.56 mmol), and the resulting solution was stirred in a cooling bath for 20 minutes. Afterwards, the solution was concentrated and placed under vacuum to provide 3',6'-diacetoxy-2',7'-difluoro-3-oxo-3H-spiro[isobenzofuran-1,9'-xanth...
example 2
[0121] Bis(acetoxymethyl)2,2'-((4-(3',6'-diacetoxy-2',7'-difluoro-3-oxo-3H-spiro[isobenzo Synthesis of furan-1,9'-xanthene]-5-formylamino)-2-methoxyphenyl)azanediyl)diacetic acid
[0122] For the synthesis of diacetate bis(acetoxymethyl) 2,2'-((4-(3',6'-diacetoxy-2',7'-difluoro-3-oxo-3H- The reaction scheme for spiro[isobenzofuran-1,9'-xanthene]-5-carboxamido)-2-methoxyphenyl)azanediyl)ester is shown in Figure 13A and Figure 13B middle. 3',6'-diacetoxy-2',7'-difluoro-3-oxo-3H-spiro[isobenzofuran-1,9'-xanthene]-5-carboxylic acid (283 mg, 0.570 mmol) was suspended in 20 mL of anhydrous DCM and the suspension was cooled in an ice-water bath. Triethylamine (79 μL, 0.56 mmol) was added to the suspension, followed by isobutyl chloroformate (88 μL, 0.67 mmol), and the resulting solution was stirred in a cooling bath for 20 minutes. The solution was then concentrated and subjected to vacuum to provide 3',6'-diacetoxy-2',7'-difluoro-3-oxo-3H-spiro[isobenzofuran-1,9'- Xanthene]-...
example 3
[0125] Synthesis of bis(acetoxymethyl)-2,2'-((5-amino-2-methoxyphenyl)azanediyl) diacetate
[0126] The reaction scheme for the synthesis of bis(acetoxymethyl)-2,2'-((5-amino-2-methoxyphenyl)azanediyl) diacetate is shown in Figure 14 middle. Dimethyl 2,2'-((2-methoxyphenyl)azanediyl)diacetate (315 mg; 1.178 mmol) was dissolved in 96% sulfuric acid (0.86 mL; 15 mmol) and cooled in an ice-water bath The solution was cooled for 15 minutes. Powdered potassium nitrate (119 mg; 1.17702 mmol) was added to the mixture in portions over 15 minutes. The reaction mixture was stirred in the cooling bath for 2 h, then quenched with ice, and the product was extracted with EtOAc (3 x 50 mL). The combined extracts were washed with saturated NaHCO3, water, brine, washed with Na 2 SO 4 Dry and evaporate. The crude material was purified on a silica gel column with an ethyl acetate-hexane gradient (0-40%) to afford 2,2'-((2-methoxy-5-nitrophenyl)azanediyl) Dimethyl diacetate (220 mg, 60%)....
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com