Preparation method of tricyclic EGFR kinase inhibitor
A solvate and organic solvent technology, applied in the field of medicinal chemistry, to achieve high yield and purity, easy operation, and easy purification
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
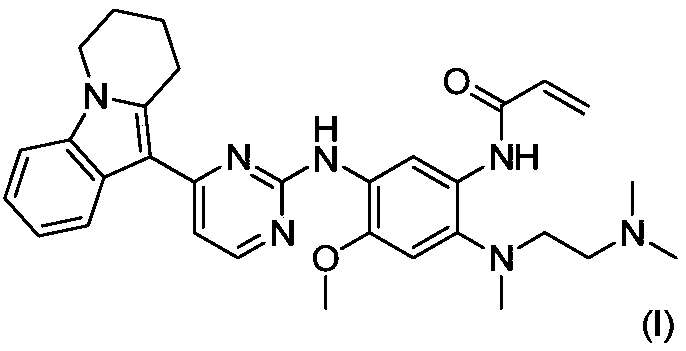
[0041] Example 1N-(2-((2-(dimethylamino)ethyl)(methyl)amino)-4-methoxy-5-((4-(6,7,8,9-tetrahydro Preparation of pyrido[1,2-a]indol-10-yl)pyrimidin-2-yl)amino)phenyl)acrylamide
[0042]
[0043] Step 1: Synthesis of 10-(2-chloropyrimidin-4-yl)-6,7,8,9-tetrahydropyrido[1,2-a]indole
[0044]
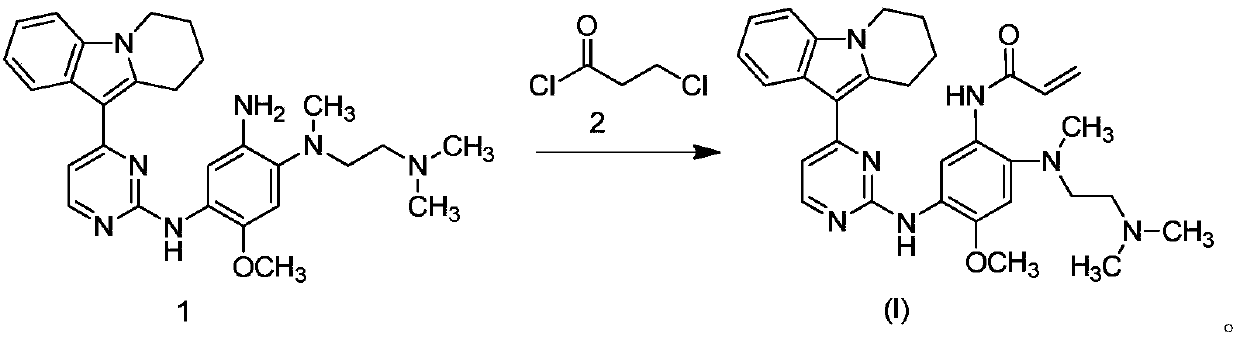
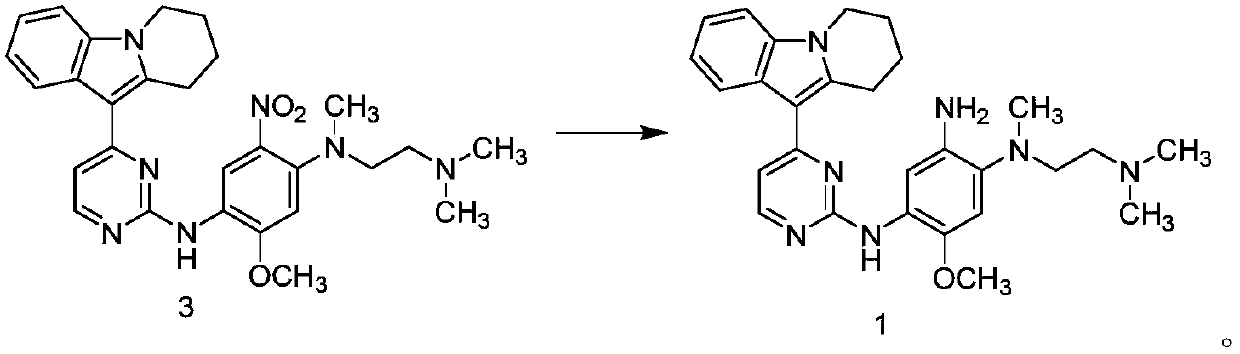
[0045] In a 100L vertical jacketed glass reactor, add ethylene glycol dimethyl ether (39.15kg) and 2,4-dichloropyrimidine (3.915kg), cool the solid-liquid mixture to below 10°C, and add anhydrous Aluminum chloride (3.855kg), the rate of addition is controlled, and the temperature is not higher than 30°C. After the addition is complete, stir at 25±5°C for 30 minutes, then add 6,7,8,9-tetrahydropyrido[1,2-a]indole (4.500kg), heat up, and react 3 at 60±5°C After 1 hour, the HPLC monitors that the content of 6,7,8,9-tetrahydropyrido[1,2-a]indole does not exceed 1.0%, and the reaction is determined to be complete. Cool the reaction solution below 25°C, add purified water (90.0kg), stir,...
Embodiment 2
[0061] Example 2: N-(2-((2-(dimethylamino)ethyl)(methyl)amino)-4-methoxy-5-((4-(6,7,8,9- Synthesis of Tetrahydropyrido[1,2-a]indol-10-yl)pyrimidin-2-yl)amino)phenyl)acrylamide
[0062]
[0063] The preparation method was the same as the preparation method in Step 5 of Example 1, except that N,N-dimethylacetamide was replaced by N,N-dimethylformamide to obtain the title compound with a purity of 69%.
[0064] N-(2-((2-(dimethylamino)ethyl)(methyl)amino)-4-methoxy-5-((4-(6,7, 8,9-tetrahydropyrido[1,2-a]indol-10-yl)pyrimidin-2-yl)amino)phenyl)acrylamide has higher yield and purity, mild reaction conditions, and purification Easy, stable process, easy to operate, environmentally friendly, and able to meet industrial-scale production and application.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com