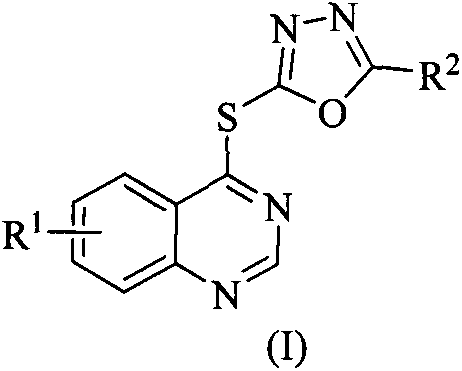
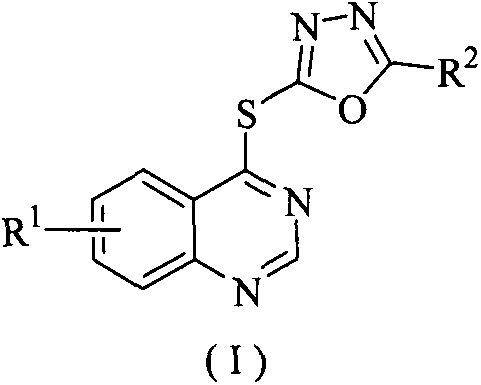
Quinazoline-containing 1,3,4-oxadiazole derivative, and preparation method and application threreof
A technology of oxadiazoles and quinazolines, which is applied in the field of prevention and control of plant pathogenic bacteria, can solve the problems of general activity against plant pathogenic bacteria, and achieve the effect of outstanding inhibitory activity and obvious application value
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
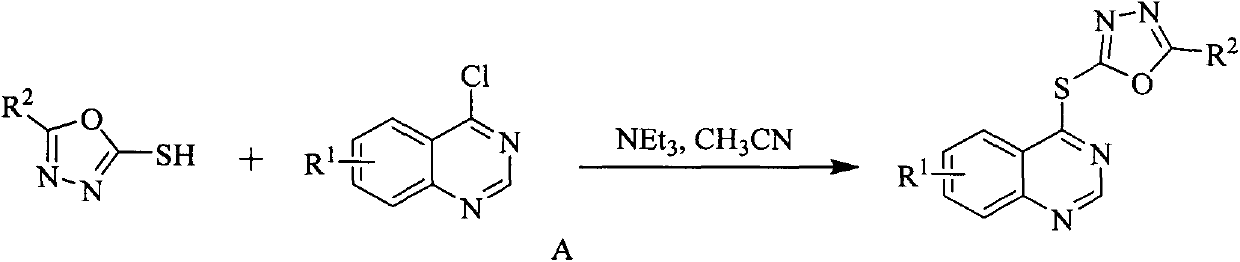
[0037] Example 1: Synthesis of 2-phenyl-5-((quinazolin-4-yl)thio)-1,3,4-oxadiazole (Ia):
[0038] Add 2-phenyl-5-mercapto-1,3,4-oxadiazole (2.81mmol), 4-chloroquinazoline (2.81mmol), triethylamine (8.42mmol) and 40mL acetonitrile into a 50mL three-necked flask , after stirring at room temperature for about 15 minutes, heated to reflux, TLC followed the reaction process (V 石油醚 :V 乙酸乙酯 =3:1), after the raw material point disappeared, stop the reaction. After cooling to room temperature, the solvent was distilled off under reduced pressure, and recrystallized from ethanol to obtain white solid Ia.
[0039] Compound Ib-Iw is synthesized sequentially according to the method of embodiment one, and the physicochemical properties and mass spectral data of the synthetic quinazoline-containing 1,3,4-oxadiazole derivatives (Ia-Iw) are shown in Table 2, NMR hydrogen Spectrum ( 1 H NMR) and carbon spectrum ( 13 C NMR) data are shown in Table 3:
[0040] Table 2 The physicochemical pr...
Embodiment 2
[0046]Embodiment two: the antibacterial activity of 1,3,4-oxadiazole derivatives (Ia-Iw) containing quinazoline
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com