Preparation method of 2D oxygen reduction catalyst Fe3O4@FeNC nanosheet
A technology of catalysts and nanosheets, applied in chemical instruments and methods, physical/chemical process catalysts, chemical/physical processes, etc., can solve problems such as poor stability, limitations, and high prices, and achieve high stability and strong innovation Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
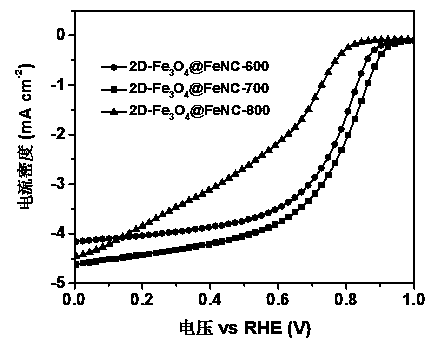
Image
Examples
Embodiment 1
[0028] In this example, graphene oxide was used as a template to construct 2D-Fe 3 o 4 @FeNC - T two-dimensional composite materials.
[0029] The details of the solution will be described in detail below in conjunction with specific embodiments and accompanying drawings, as follows.
[0030] (1) Weigh a certain mass of graphene oxide and place it in a round bottom flask, add a certain amount of deionized water, ultrasonically disperse for 15 minutes, and then magnetically stir for 15 minutes to obtain a uniform dispersion of 0.25 mg / mL.
[0031] (2) Add a certain amount of pyrrole monomer into the above solution, and continue stirring for 15 minutes, wherein the weight ratio of the pyrrole monomer to graphene oxide is 20:1.
[0032] (3) Weigh ferric chloride and dissolve it in deionized water, and add it dropwise to the above solution. After 24 hours of polymerization, filter with suction and wash with deionized water to obtain a graphene-polypyrrole two-dimensional compos...
Embodiment 2
[0039] (1) Weigh a certain amount of graphene oxide into a round bottom flask, add a certain amount of deionized water, ultrasonically disperse for 15 minutes, and then magnetically stir for 15 minutes to obtain a uniform dispersion of 0.1 mg / mL.
[0040] (2) Add a certain amount of pyrrole monomer into the above solution, and continue stirring for 15 minutes, wherein the weight ratio of the pyrrole monomer to graphene oxide is 10:1.
[0041] (3) Weigh ferric chloride and dissolve it in deionized water, and add it dropwise to the above solution. After 24 hours of polymerization, filter with suction and wash with deionized water to obtain a graphene-polypyrrole two-dimensional composite. In the polymerization process, pyrrole single The weight ratio of body to ferric chloride is 1:5.
[0042](4) Disperse the graphene-polypyrrole two-dimensional composite into an ethanol solution, add a certain amount of ferric chloride, and stir at 60°C for 60 minutes, and then spin dry. In the...
Embodiment 3
[0048] (1) Weigh a certain mass of graphene oxide and place it in a round bottom flask, add a certain amount of deionized water, ultrasonically disperse for 15 minutes, and then magnetically stir for 15 minutes to obtain a uniform dispersion of 0.5 mg / mL.
[0049] (2) Add a certain amount of pyrrole monomer into the above solution, and continue stirring for 15 minutes, wherein the weight ratio of the pyrrole monomer to graphene oxide is 30:1.
[0050] (3) Weigh ferric chloride and dissolve it in deionized water, and add it dropwise to the above solution. After 24 hours of polymerization, filter with suction and wash with deionized water to obtain a graphene-polypyrrole two-dimensional composite. In the polymerization process, pyrrole single The weight ratio of body to ferric chloride is 1:6.
[0051] (4) Disperse the graphene-polypyrrole two-dimensional composite into an ethanol solution, add a certain amount of ferric chloride, and stir at 60°C for 60 minutes, and then spin d...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com