Medical use of optical isomer of amine compound in treating pain
A technology of amine compounds and compounds, applied in the field of medical application of optical isomers of amine compounds to treat pain, can solve the problems of central sedation adverse reactions, cardiotoxicity, dependence and addiction, and achieve excellent analgesia Effect, little toxicity effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0052] Embodiment 1. Mouse acetic acid writhing method evaluates the analgesic effect of amoxetine
[0053] 1. Experimental animals:
[0054] KM mouse, male, SPF grade, 22-25g,
[0055] The animals were purchased from the Experimental Animal Center of the Academy of Military Medical Sciences and bred in the Experimental Animal Center of the Academy of Military Medical Sciences.
[0056] 2. Test sample:
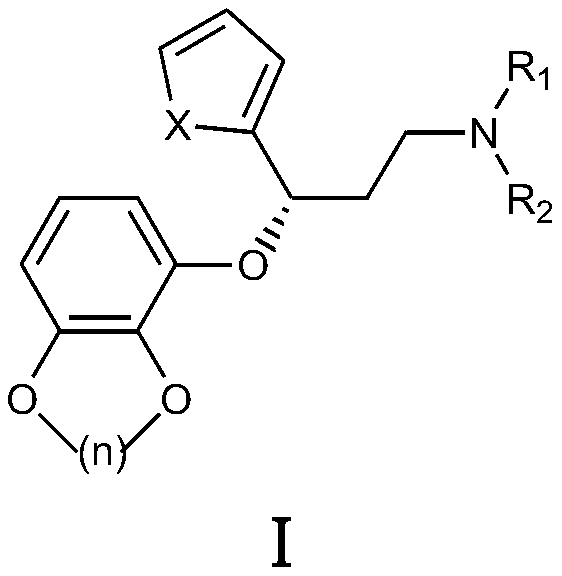
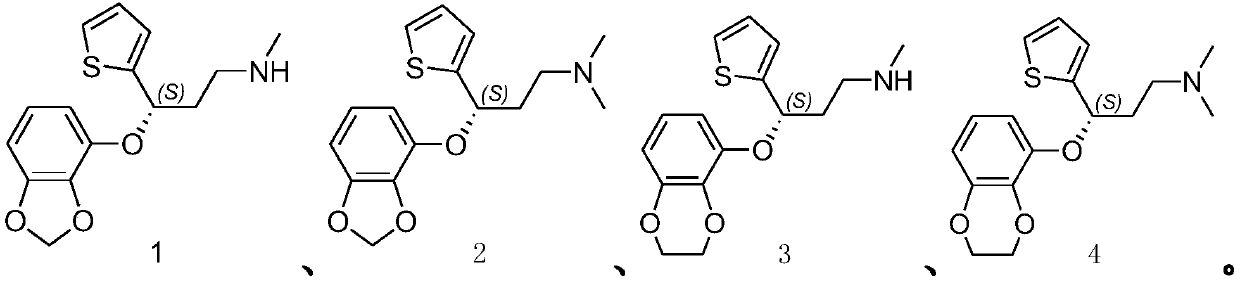
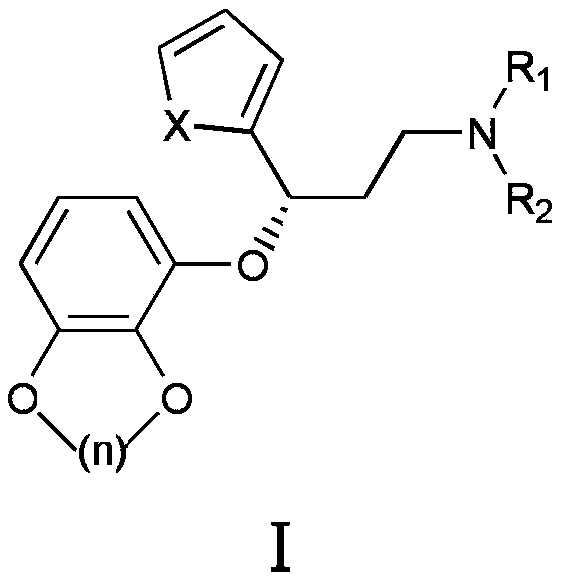
[0057] Amoxetine, the structural formula is According to the method of embodiment 5 in the patent CN102850335A, it is prepared;
[0058] Duloxetine hydrochloride (Zhejiang Haixiang Pharmaceutical Co., Ltd.).
[0059] 3. Experimental method:
[0060] 30 minutes after the mice were orally administered the test drug amoxetine or the positive drug duloxetine, each mouse was intraperitoneally injected with 2% acetic acid aqueous solution 0.1ml / 10g, and the writhing of each mouse was observed and recorded within 5-20 minutes The number of times, that is, the body twisting or...
Embodiment 2
[0066] Embodiment 2. Mouse formalin method evaluates the analgesic effect of amoxetine
[0067] 1. Experimental animals:
[0068] KM mouse, male, SPF grade, 22-25g,
[0069] The animals were purchased from the Experimental Animal Center of the Academy of Military Medical Sciences and bred in the Experimental Animal Center of the Academy of Military Medical Sciences.
[0070] 2. Test sample:
[0071] Amoxetine, prepared according to the method of Example 5 in the patent CN102850335A;
[0072] Duloxetine hydrochloride (Zhejiang Haixiang Pharmaceutical Co., Ltd.).
[0073] 3. Experimental method:
[0074] 30 minutes after the mice were orally administered the test drug amoxetine or the positive drug duloxetine, each mouse was subcutaneously injected with 5% formalin aqueous solution 0.02ml on the right paw of each mouse, and observed and recorded for 0-5 minutes respectively (acute phase) The times of licking the feet of each mouse in the two time periods of 10-25 minutes ...
Embodiment 3
[0080] Embodiment 3. Rat sciatic nerve branch selective injury (SNI) model evaluates the analgesic effect of amoxetine
[0081] 1. Experimental animals:
[0082] SD rat, male, SPF grade, 180-220g,
[0083] The animals were purchased from the Experimental Animal Center of the Academy of Military Medical Sciences and bred in the Experimental Animal Center of the Academy of Military Medical Sciences.
[0084] 2. Test sample:
[0085] Amoxetine, prepared according to the method of Example 5 in the patent CN102850335A;
[0086] Duloxetine hydrochloride (Zhejiang Haixiang Pharmaceutical Co., Ltd.).
[0087] 3. Experimental method:
[0088] Rats were anesthetized by intraperitoneal injection of pentobarbital sodium (50mg / kg), and the rats were fixed on the operating table in a lateral position. After disinfection with iodine, the skin on the upper edge of the mouse's right hind limb was cut, the muscles were separated, and the trunk of the sciatic nerve was exposed. and three ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com