Synthesis method of dichlorophenol
A synthesis method and technology of dichlorophenol are applied in the synthesis of 2,5-dichlorophenol, 2,4-dichlorophenol or 3,4-dichlorophenol, and in the field of synthesis of dichlorophenol, and can solve the problem of production environment The problems of strong odor and harsh reaction conditions can achieve the effect of less three wastes, low production cost and mild reaction conditions.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0023] The invention provides a kind of synthetic method of dichlorophenol, comprising the following steps:
[0024] S1, subjecting 1,4-dichlorobenzene to an alkylation reaction to obtain 1,4-dichloro-2-isopropylbenzene;
[0025] Or subject 1,3-dichlorobenzene to an alkylation reaction to obtain 1,3-dichloro-4-isopropylbenzene;
[0026] Or subject 1,2-dichlorobenzene to an alkylation reaction to obtain 1,2-dichloro-4-isopropylbenzene;
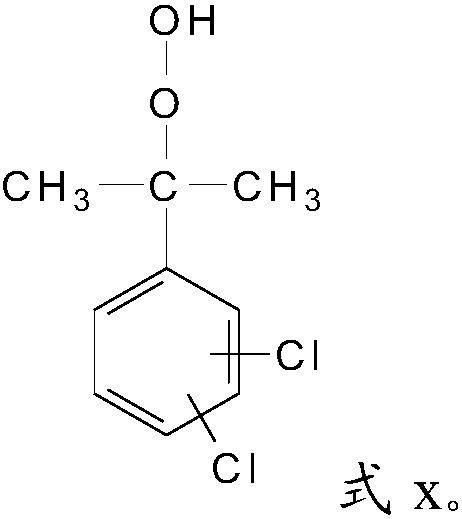
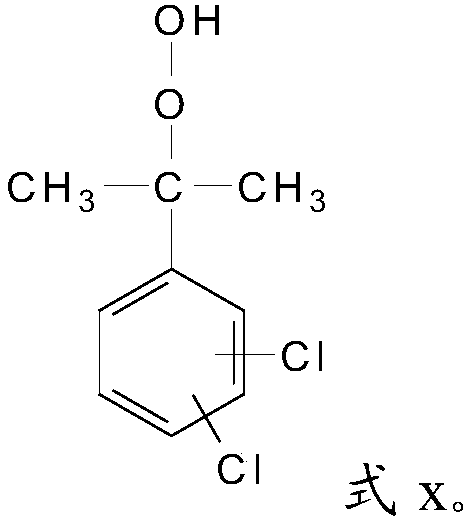
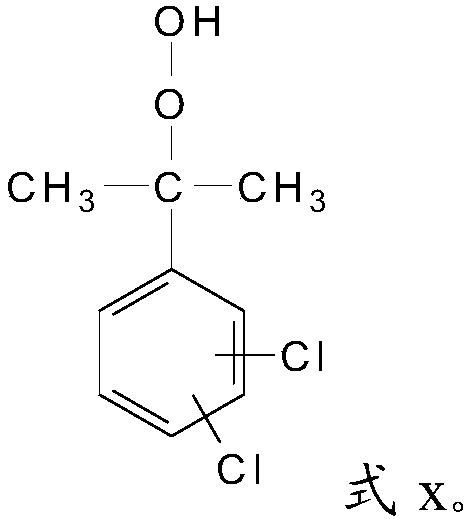
[0027] S2, the alkyl in the 1,4-dichloro-2-isopropylbenzene, the alkyl in the 1,3-dichloro-4-isopropylbenzene or 1,2-dichloro-4-isopropyl Alkyl groups in benzene are oxidized to obtain dichloroperoxides with a structure of formula x;
[0028] S3. Catalyzing and decomposing the dichloroperoxide respectively to obtain dichlorophenol and acetone;
[0029]
[0030] The synthesis method of dichlorophenol provided by the invention has the advantages of high product purity, less waste, simple and easy preparation, low cost, etc., and is suitable...
Embodiment 1
[0049] Take 294g of 1,4-dichlorobenzene, 170g of 2-chloropropane and 30g of aluminum trichloride into the autoclave, raise the temperature to 60°C, raise the pressure to 0.5MPa, keep the pressure for 5 hours, cool down to 25°C after the reaction is complete, and release After pressing, the obtained reaction material was washed with 200 g of water until neutral, and then dried to obtain 348.2 g of 1,4-dichlorocumene. Add 10g of 5wt% sodium carbonate solution to the oxidation reactor, pressurize the air to 0.5MPa pressure, raise the temperature to 110°C, transfer the obtained 1,4-dichloroisopropylbenzene, and oxidize it to 1,4-dichloroisopropyl Benzene peroxide, the temperature was lowered to obtain 390.6 g of the peroxide.
[0050] Add 50g acetone, 20g gained 1,4-dichlorocumene peroxide, 10g catalyst macroporous sulfonic acid resin (aperture 1mm) in the four-necked flask equipped with condenser and stirrer, put the four-necked flask Put it into a constant temperature water bat...
Embodiment 2
[0052] Take 294g of 1,3-dichlorobenzene, 170g of 2-chloropropane and 30g of solid phosphoric acid catalyst into the autoclave, raise the temperature to 50°C, raise the pressure to 0.6MPa, keep the pressure for 5 hours, cool down to 25°C after the reaction is complete, and release the pressure After completion, the obtained reaction material was washed with 200 g of water until neutral, and then dried to obtain 352.2 g of 1,3-dichlorocumene. Add 10g of 5% sodium carbonate solution to the oxidation reactor, pressurize the air to 0.5MPa, raise the temperature to 110°C, transfer the obtained 1,3-dichloroisopropylbenzene, and oxidize it into 1,3-dichloroisopropyl Benzene peroxide, the peroxide is obtained by cooling.
[0053] Add 50g acetone, 20g gained 1,3-dichlorocumene peroxide, 10g catalyst macroporous sulfonic acid resin (aperture 1.5mm) in the four-necked flask that is equipped with condenser and stirrer, the four-necked flask Put it into a constant temperature water bath, r...
PUM
| Property | Measurement | Unit |
|---|---|---|
| pore size | aaaaa | aaaaa |
| pore size | aaaaa | aaaaa |
| pore size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com