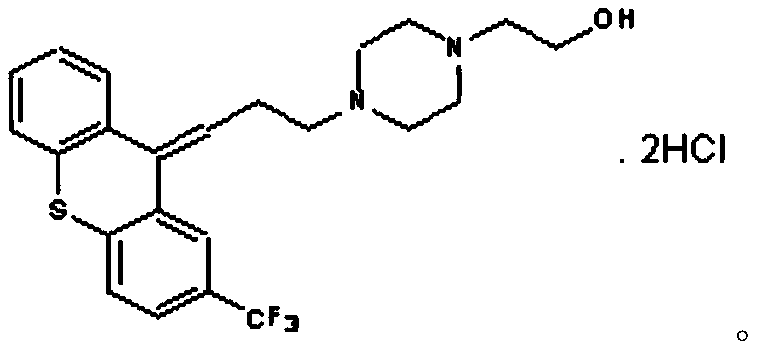
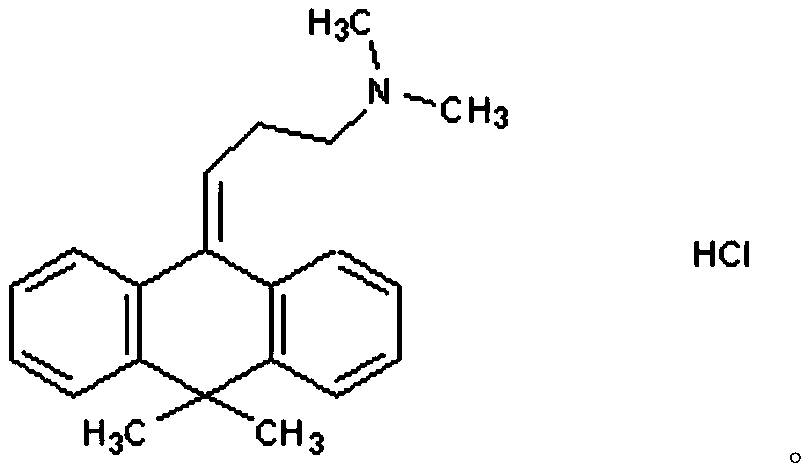
Flupentixol and melitracen tablets and preparation method thereof
A technology of melitracen tablets and flupenthixol, which is applied in the field of pharmaceutical preparations, can solve the problems of not containing stabilizers and cannot guarantee product stability, so as to reduce the level of impurities, ensure product safety and effectiveness, The effect of enhancing stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0054] A flupenthixol melitracen tablet, wherein the mass percentages of flupenthixol hydrochloride, melitracen hydrochloride, antioxidant, filler, disintegrant, binder, and lubricant are respectively 0.48 %, 9.30%, 0.08%, 82.69%, 1.49%, 4.96%, 0.99%, the quality of each component is shown in Table 1.
[0055] The preparation method of tablet described in the present embodiment is:
[0056] (1) Preparation of Flupenthixol Granules
[0057] Dissolve flupenthixol hydrochloride and dibutyl hydroxytoluene in 80% ethanol-water solution, stir until clear; put anhydrous lactose, microcrystalline cellulose, and hydroxypropyl cellulose into a high-speed stirring granulator, add hydrochloric acid 80% ethanol-water solution of flupenthixol and dibutyl hydroxytoluene, stirring and shearing granulation; fluidized bed drying, granulation to obtain flupenthixol granules;
[0058] (2) Preparation of melitracen granules
[0059] Put melitracen hydrochloride, anhydrous lactose, microcrystall...
Embodiment 2
[0066] A flupenthixol melitracen tablet, wherein the mass percentages of flupenthixol hydrochloride, melitracen hydrochloride, antioxidant, filler, disintegrant, binder, and lubricant are respectively 0.48 %, 9.19%, 0.82%, 81.68%, 1.96%, 4.41%, 1.47%, the quality of each component is shown in Table 1.
[0067] The preparation method of tablet described in the present embodiment is:
[0068] (1) Preparation of Flupenthixol Granules
[0069] Dissolve flupenthixol hydrochloride and propyl gallate in 75% ethanol-water solution, stir until clear; put anhydrous lactose, starch, and hydroxypropyl cellulose into a high-speed stirring granulator, add flupenthixol hydrochloride With 75% ethanol-water solution of propyl gallate, stirring and shearing granulation; fluidized bed drying, granulation to obtain flupentixol granules;
[0070] (2) Preparation of melitracen granules
[0071] Put melitracen hydrochloride, anhydrous lactose, starch, and hydroxypropyl cellulose into a high-speed...
Embodiment 3
[0074] A flupenthixol melitracen tablet, wherein the mass percentages of flupenthixol hydrochloride, melitracen hydrochloride, antioxidant, filler, disintegrant, binder, and lubricant are respectively 0.48 %, 9.20%, 0.65%, 81.81%, 1.96%, 4.42%, 1.47%, the quality of each component is shown in Table 1.
[0075] The preparation method of tablet described in the present embodiment is:
[0076] (1) Preparation of Flupenthixol Granules
[0077] Dissolve flupentixol hydrochloride and tert-butyl p-hydroxyanisole in 75% ethanol-water solution, stir until clear; put calcium hydrogen phosphate, microcrystalline cellulose, and hypromellose into a high-speed stirring granulator , adding flupenthixol hydrochloride and 75% ethanol-water solution of tert-butyl-p-hydroxyanisole, stirring and shearing to granulate; fluidized bed drying, granulation to obtain flupentixol granules;
[0078] (2) Preparation of melitracen granules
[0079] Put melitracen hydrochloride, calcium hydrogen phosphat...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com