Preparation method for tofacitinib citrate
A technology of tofacitinib and citric acid, applied in the field of drug synthesis, can solve the problems of complicated post-processing, high cost, unstable product purity and the like, and achieves the effects of high reaction yield, simple post-processing and few by-products
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
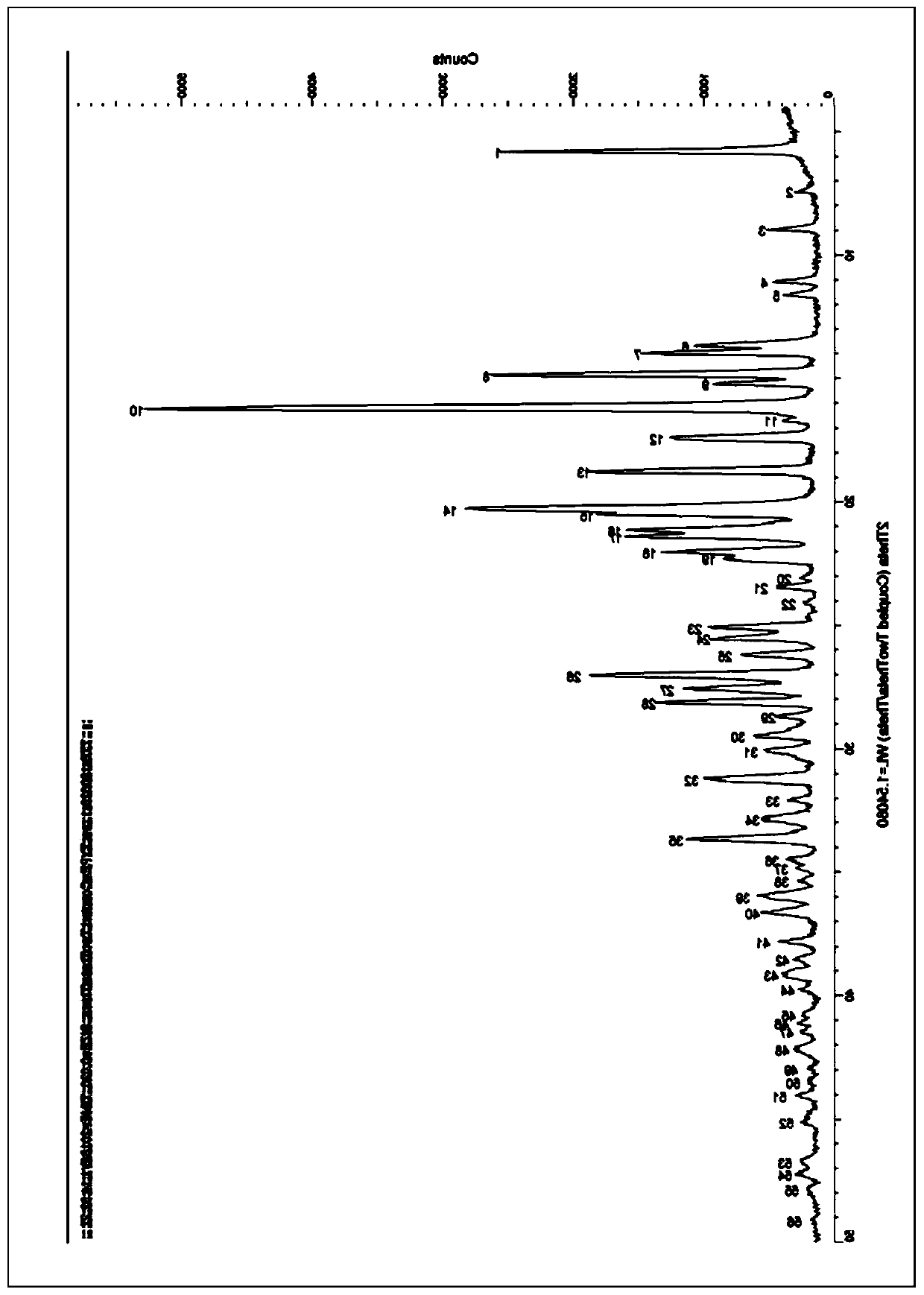
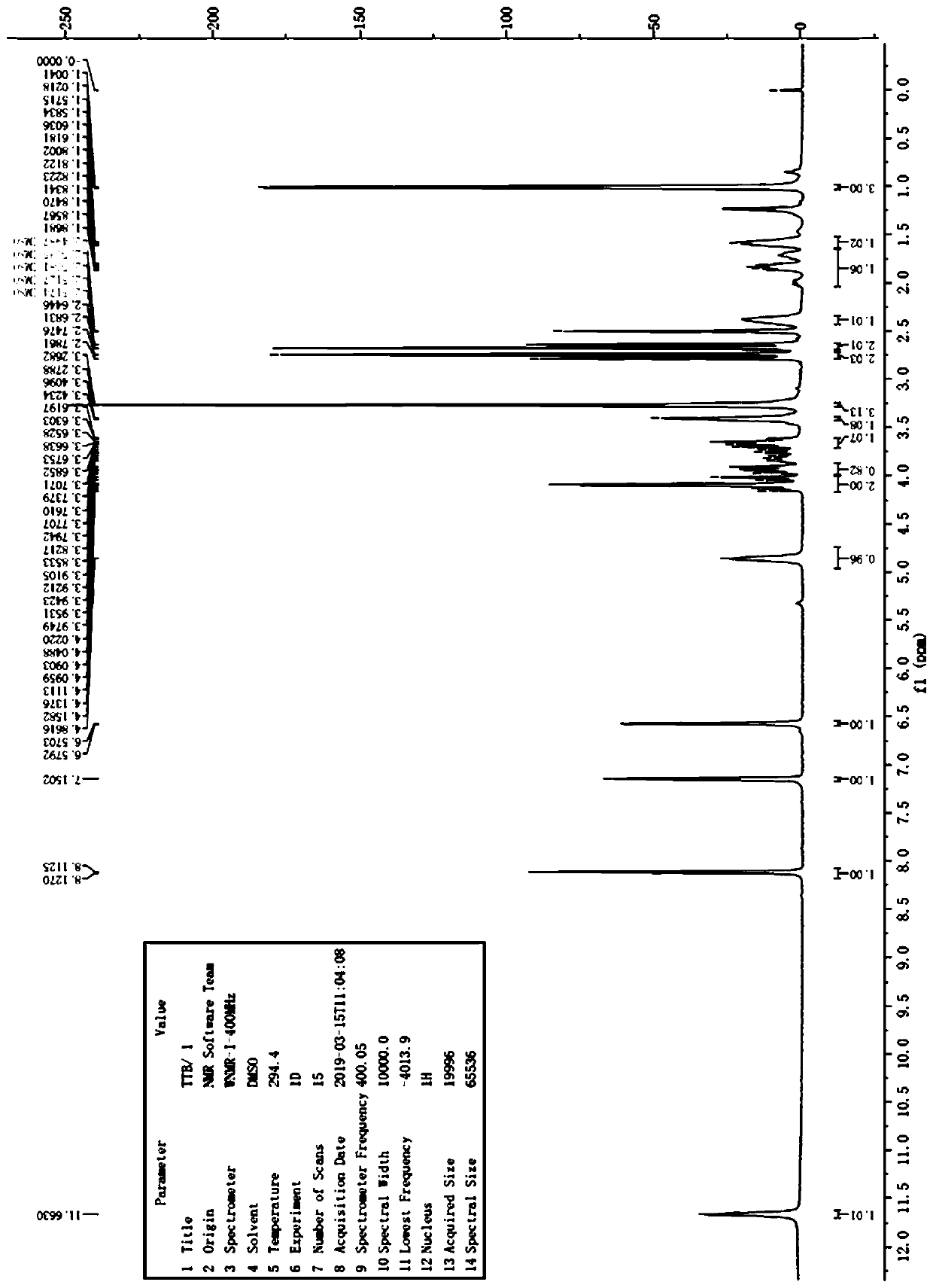
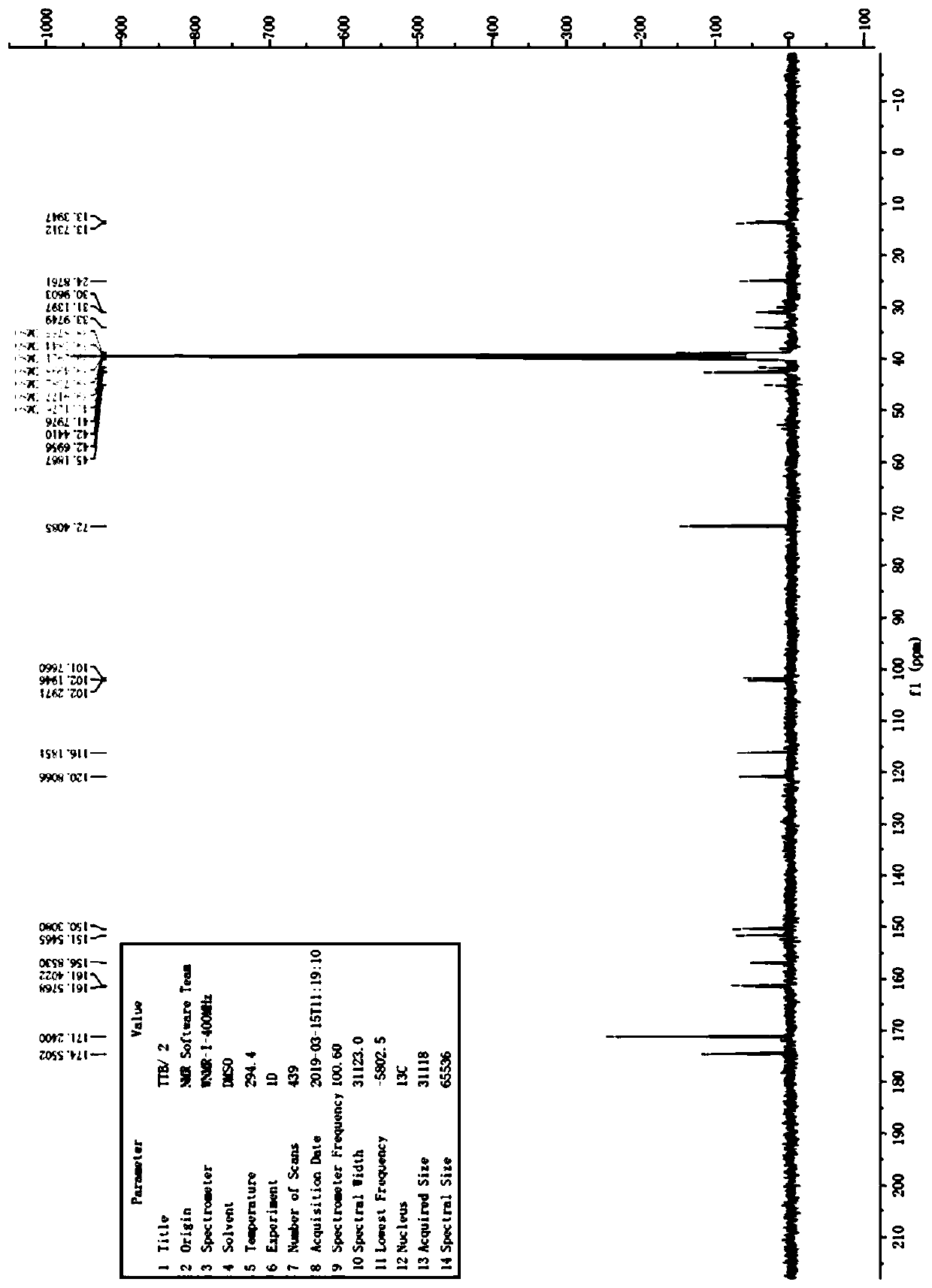
Image
Examples
Embodiment 1
[0045] At room temperature, 12.0Kg (35.8mol) ((3R,4R)-1-benzyl-4-methyl-piperidin-3-yl)-methyl-(7H-pyrrolo[2,3-D] Pyrimidin-4-yl)-amine and 1mol / L hydrochloric acid aqueous solution were added to the hydrogenation kettle, and 1.2Kg of palladium carbon with a water content of 50% was added, and hydrogenation was carried out at room temperature under 0.2-0.4MPa hydrogen pressure for 4-6 hours. After the reaction was completed, Palladium carbon was filtered off, concentrated to remove water and dried to obtain ((3R,4R)-4-methyl-piperidin-3-yl)-methyl-(7H-pyrrolo[2,3-D]pyrimidine-4 -base)-amine salt 11.1Kg, yield 97.5%.
[0046] Add the above-mentioned amine salt and 19.9Kg (130.7mol) DBU into ethanol, stir and heat up to 35°C, add 7.9Kg (69.8mol) of ethyl cyanoacetate dropwise and react for 4-8 hours. After the reaction is completed, add aqueous sodium carbonate solution to quench extinguished, stirred and cooled to crystallize, obtained tofacitinib 10.0Kg after suction filtrati...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com