Synthesis method of high-purity phthalic acid n-pentyl isoamyl ester
A technology of n-pentyl isopentyl ester and phthalic acid, which is applied to the preparation of carboxylic acid esters, chemical instruments and methods, and the preparation of carboxylic acid halides, to achieve easy control of the reaction, mild reaction conditions, and simple requirements for reaction equipment Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
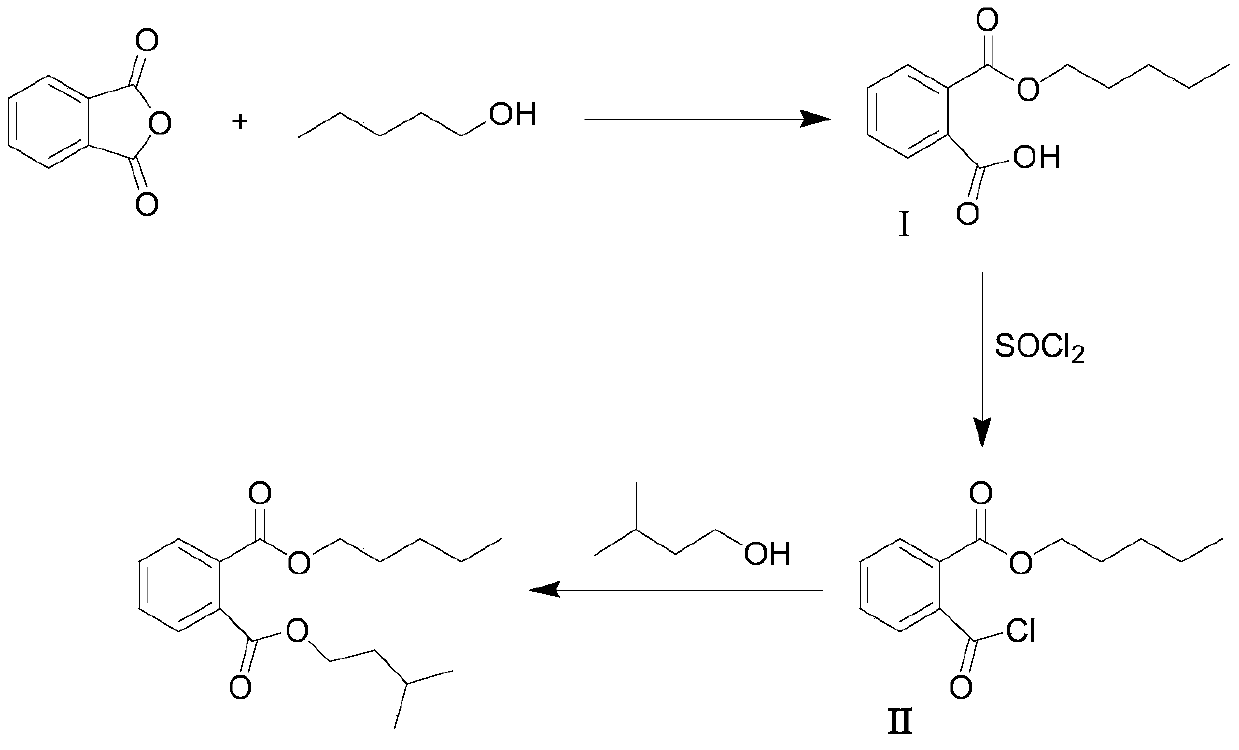
[0023] A process for synthesizing n-pentyl isopentyl phthalate, the steps are as follows:
[0024] (1) Prepare a 3L glass reactor, first add n-amyl alcohol (595g, 6.75mol), phthalic anhydride (1.0kg, 6.75mol), 1.0L pyridine in sequence, stir and dissolve, there is an exothermic phenomenon, heat up, The internal temperature is controlled at 60-65°C for 3 hours of ripening. After the reaction is over, add the reaction solution into 2L of water, add concentrated hydrochloric acid dropwise while stirring, adjust the pH value of the reaction solution to 1-2, and then use dichloromethane (3L×3) extraction, the combined organic phase, the organic phase was successively washed with 1N HCl solution (3L×2), water (3L×1), saturated sodium chloride solution (3L×1), dried over magnesium sulfate (200g), Concentrate to obtain 1.5kg white solid intermediate I, yield: 94%.
[0025] (2) Prepare a 5L glass reaction kettle and tail gas recovery device: first add 2.25L thionyl chloride and 1.5kg ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com