Method for preparing 4,5-dinitroimidazole using microchannel reactor
A microchannel reactor, technology of dinitroimidazole, applied in chemical instruments and methods, organic chemistry, chemical/physics/physicochemical processes, etc., can solve problems such as increased production cost, unsuitable temperature control, process safety issues, etc. To achieve the effect of convenient installation and dismantling, lower experiment cost and low energy consumption
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
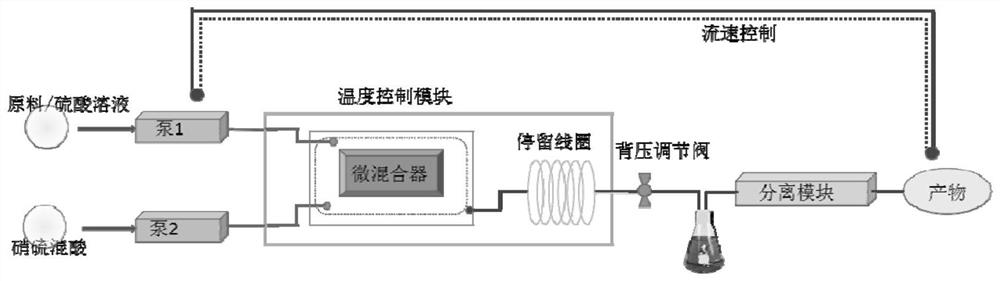
Image
Examples
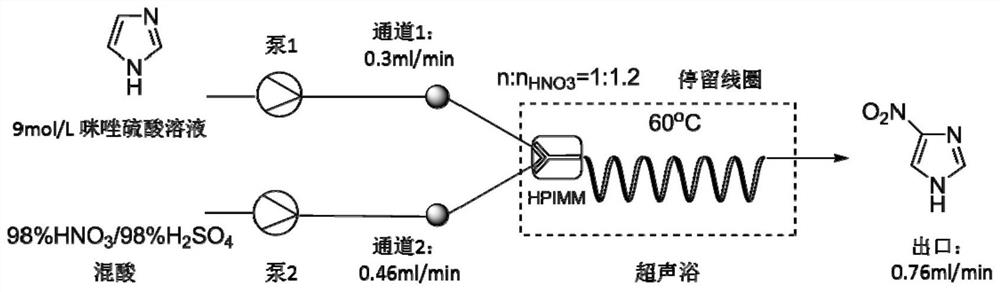
Embodiment 1
[0043] Adopting 98% vitriol oil and 98% nitric acid preparation molar ratio is the mixed acid of 2:1, regulates the volumetric flow rate of mixed acid and imidazole sulfuric acid solution, the mol ratio of control nitric acid and imidazole is 1.1, at total flow rate is 0.76mL / min ( The flow velocity of imidazole sulfuric acid solution is 0.3mL / min) under the condition that carries out temperature influence experimental investigation, and 4-nitroimidazole productive rate is shown in Table 1 and Figure 4 .
[0044] Table 1
[0045]
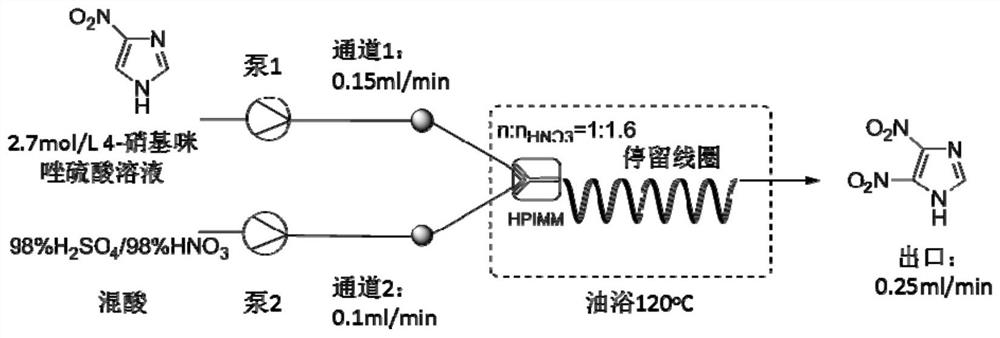
Embodiment 2
[0047] Process is the same as embodiment 1, and fixed temperature is 60 ℃, only changes the flow velocity of mixed acid, carries out relevant experiment to the productive rate of 4-nitroimidazole to the mol ratio of nitric acid and imidazole, and the results are shown in Table 2 and Figure 5 .
[0048] Table 2
[0049]
Embodiment 3
[0051] Process is with embodiment 1, only changes the mol ratio of sulfuric acid and nitric acid in mixed acid, and 4-nitroimidazole productive rate is shown in Table 3 and Figure 6 .
[0052] table 3
[0053]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com