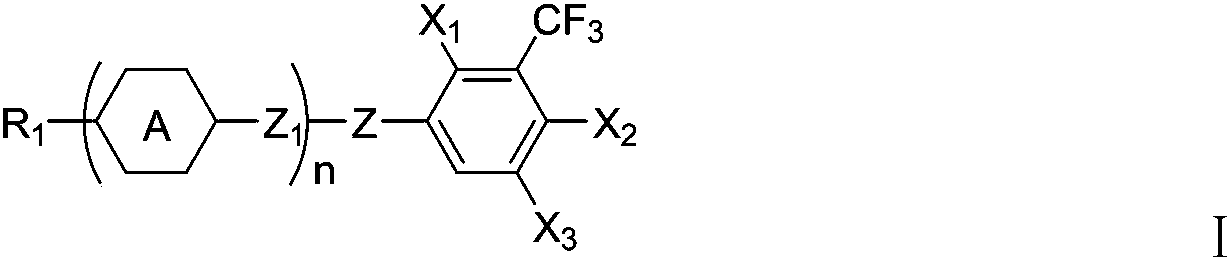
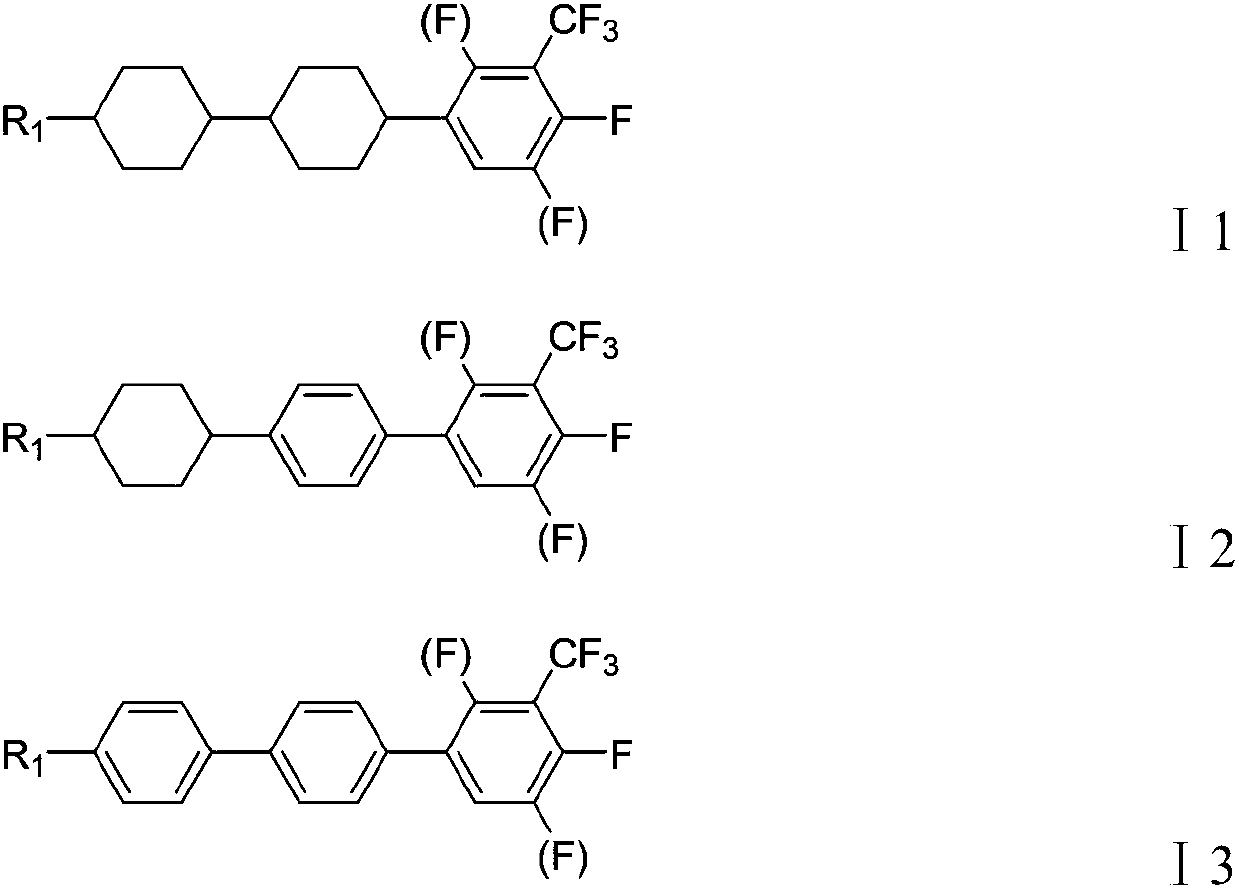
Liquid crystal compound containing lateral trifluoromethyl group, liquid crystal mixture and display device thereof
A liquid crystal compound and compound technology, applied in the direction of liquid crystal materials, chemical instruments and methods, etc., can solve the problems of low-temperature miscibility, defects in vertical dielectrics, and not suitable for the development of high-penetration liquid crystals, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
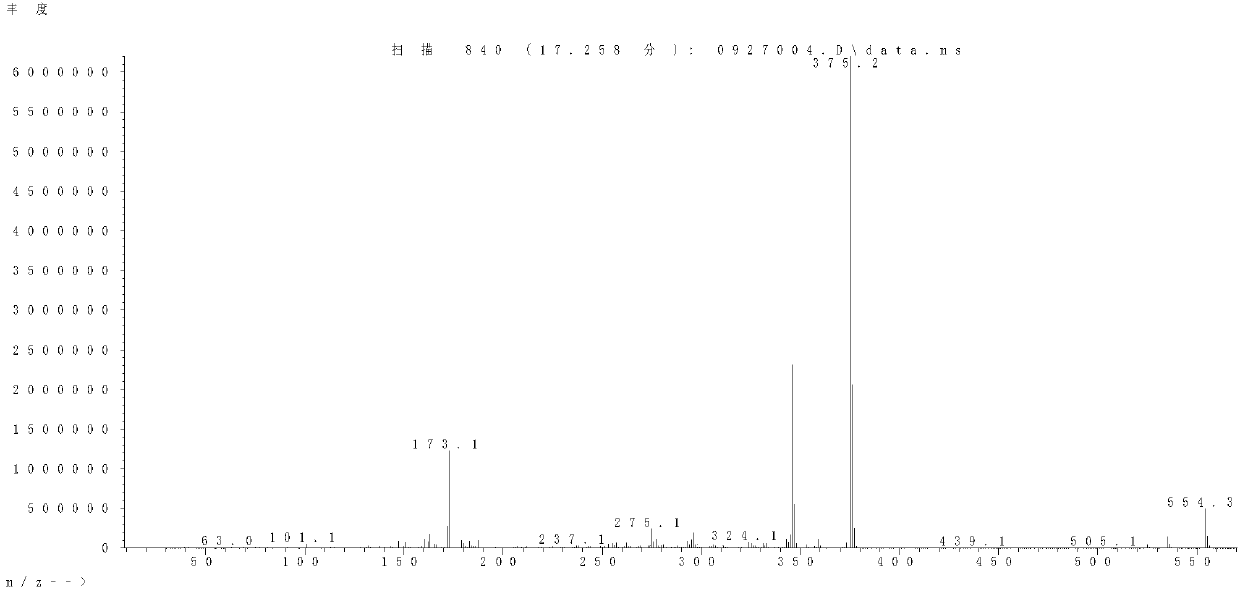
Image
Examples
Embodiment 1
[0129]
[0130] Put 4.54g (0.01mol) (1-a-1), 2.0g (0.011mol) (1-a-2), 20ml DMF, and 1.8g anhydrous potassium carbonate into a 50ml three-necked flask together, and keep stirring at 100°C Reacted for 3 hours, poured into 100nl water, extracted with toluene, washed the organic phase with water, distilled off the toluene, dissolved in petroleum ether, passed through a silica gel column, recrystallized twice with toluene + petroleum ether, and obtained 2.8g of product (1-a), Gc: 99.92 %
[0131] Δε: 23.8
[0132] Δn: 0.192
[0133] Cp: 79°C (HOST fitting)
[0134] ε ⊥ : 6.2
[0135] gamma 1 :217.
[0136] With reference to the synthetic method of Example 1, only part of the raw materials are replaced, and the following compounds can be synthesized
[0137]
[0138] Δε: 29.8
[0139] Δn: 0.182
[0140] Cp: 68°C (HOST fitting)
[0141] ε⊥: 6.6
[0142] Gamma 1:235.
Embodiment 2
[0165]
[0166] 24.2g (0.1mol) raw material (2-a-2), 27.06g (0.11mol) (2-a-1), 9.96g (0.12mol) anhydrous sodium carbonate, 150ml toluene, 150ml ethanol, and 120ml water 1000ml there-necked flask, add 0.5g of catalyst palladium tetrakistriphenylphosphine after slight boiling, and react under reflux under stirring for 5 hours.
[0167] Add 300ml of water, separate the water phase, wash the organic phase with water, evaporate the solvent to dryness, dissolve petroleum ether, pass through a silica gel column, and recrystallize petroleum ether 3 times to obtain 27.3g of white product (2-a) with a yield of 75%, Gc: 99.86%.
[0168] Δε: 15.8
[0169] Δn: 0.140
[0170] Cp: 65°C (HOST fitting)
[0171] ε⊥: 6.1.
[0172] The same synthesis method, only replacing the raw materials, can get:
[0173]
Embodiment 3
[0175]
[0176] 29g (0.12mol) (3-a-2) was dissolved in 50ml THF, 2.9g of magnesium chip was added in the 500ml there-necked bottle, 50ml THF, and the solution of (3-a-2) dissolved in 50ml THF was added dropwise under reflux, 1 Hours were added to prepare the Grignard reagent.
[0177] Add dropwise a solution of 22.2g (0.10mol) (3-a-1) dissolved in 50ml THF to the above format reagent, reflux for one hour after adding, pour into aqueous hydrochloric acid solution, add 100ml of toluene, separate the aqueous phase, and wash the organic phase with water .
[0178] Evaporate the solvent, add 150ml of toluene, add 1g of p-toluenesulfonic acid, and separate the water under reflux for about 5 hours.
[0179] Passed through a silica gel column, and recrystallized from ethanol three times to obtain 24.2 g of a white product (3-b), with a yield of 66%.
[0180] White product (3-b) 24.2g is dissolved in 200ml dehydrated alcohol, puts into palladium carbon 1g, under normal pressure hy...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com