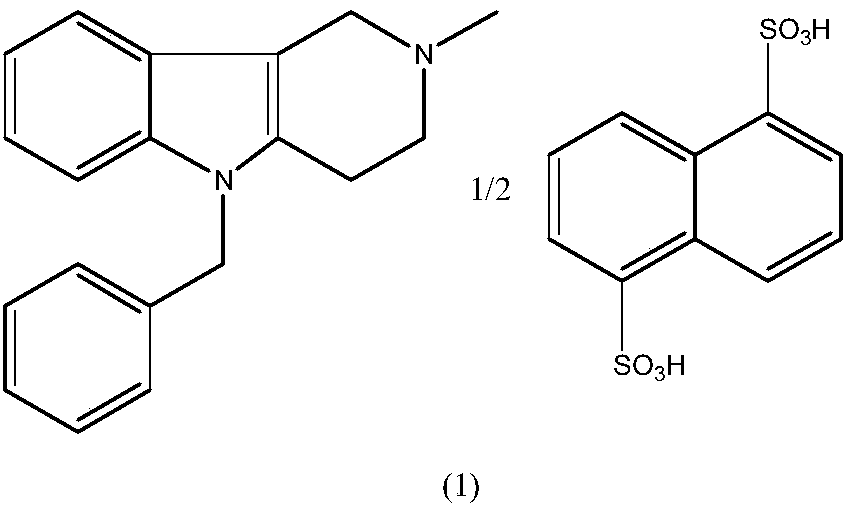
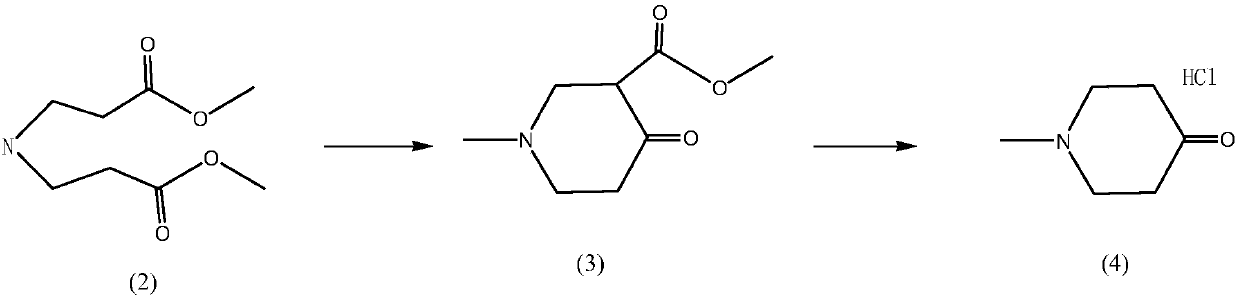
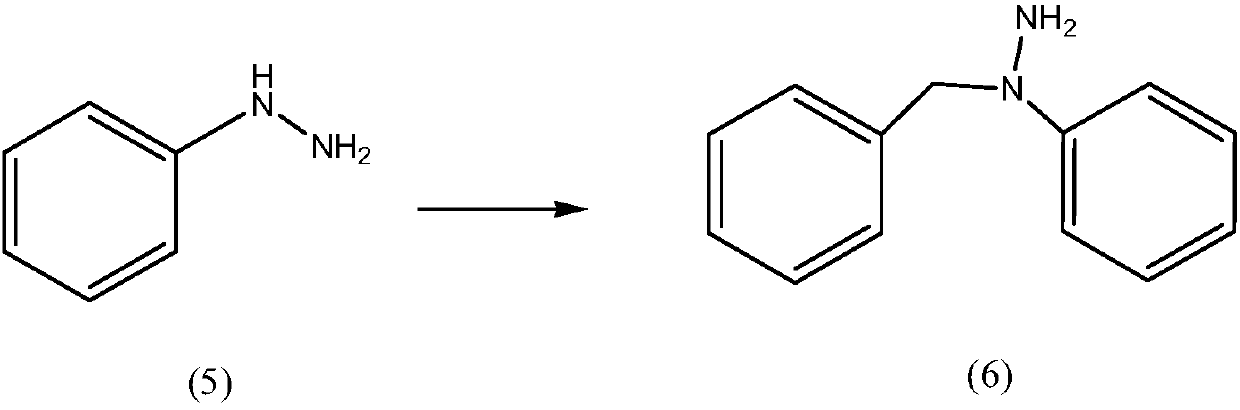
Preparation method of mebhydrolin napadisylate
A technology of heltraline naphthalene disulfonic acid and sodium naphthalene disulfonate, which is applied in the field of preparation of mehaitraline naphthalene disulfonic acid, and can solve the problems of product quality failing to meet requirements, high sulfate ion concentration, and incomplete conversion reaction and other problems, to achieve the effect of stable improvement of product quality, improvement of reaction yield, and improvement of total reaction yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] (1) Preparation of N-methyl-4-piperidone hydrochloride solution
[0025] Add 250ml of xylene and 20.0g of methylimine-N,N-dipropionate into the reaction kettle, then add 9.5g of solid sodium methoxide after cooling down, stir and heat under reflux for 4 hours until the reaction is complete. Lower the temperature of the reactants in the previous step to 40°C, add 10ml of water, stir to completely dissolve, add 30ml of concentrated hydrochloric acid, stir for 10 minutes, and let stand to separate layers (the bottom layer will have a small amount of white insoluble matter, which is a normal phenomenon). Separate the xylene layer, extract the xylene once with 30ml of concentrated hydrochloric acid, combine the hydrochloric acid layers, and heat the hydrochloric acid layer to reflux for decarboxylation for 5 hours to obtain N-methyl-4-piperidone hydrochloride solution, containing about 10.0g of product. The yield was 90%, and the solution went directly to the next step witho...
Embodiment 2
[0031] (1) Preparation of N-methyl-4-piperidone hydrochloride solution
[0032] Add 250ml of toluene and 20g of methylimine-N,N-dipropionate into the reaction kettle, then cool down and add 48.9g of 28% liquid sodium methoxide, stir and heat under reflux for 10 hours until the reaction is complete. Lower the temperature of the reactants in the previous step to 40°C, add 10ml of water, stir to completely dissolve, add 30ml of concentrated hydrochloric acid, stir for 10 minutes, and let stand to separate layers (the bottom layer will have a small amount of white insoluble matter, which is a normal phenomenon). Separate the toluene layer, extract the toluene once with 30ml of concentrated hydrochloric acid, combine the hydrochloric acid layers, and heat the hydrochloric acid layer to reflux for decarboxylation for 5 hours to obtain N-methyl-4-piperidone hydrochloride solution, containing about 9.0g of the product. The calculated yield is 81 %, the solution went directly to the ne...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com