Preparation method of doxofylline injection
A technology of doxofylline and injection, applied in the field of medicine, can solve the problems of inconvenient clinical use, increased substance content and the like, and achieve the effects of low equipment cost, good repeatability, huge social and economic benefits
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
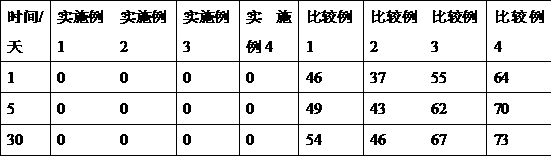
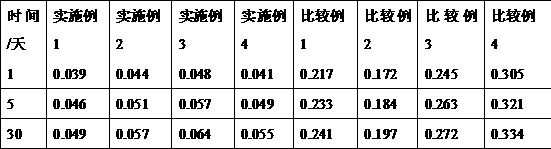
[0041] Dissolve 11.6g of hydroxypropyl-β-cyclodextrin (molecular weight: 1541) in 100mL of water for injection; adjust the pH to 2 with phosphoric acid, then add 0.25g of L-serine, stir to dissolve, and obtain serine-containing hydroxyl Propyl-β-cyclodextrin aqueous solution; then add 2g doxofylline, stir at 35°C and 300prm for 1.5h, then adjust the pH to 5.2 with sodium hydroxide solution, filter through a 0.45μm microporous membrane, and dilute with 5mL Each branch was packed in vials, and autoclaved for 30 minutes to obtain the doxofylline injection of Example 1.
Embodiment 2
[0043] Dissolve 10.2g of hydroxypropyl-β-cyclodextrin in 100mL of water for injection; adjust the pH to 2.5 with phosphoric acid, then add 0.38g of L-serine, stir to dissolve, and obtain hydroxypropyl-β-cyclodextrin containing serine Cyclodextrin aqueous solution; then add 2g doxofylline, stir at 30°C and 200prm for 1h, then adjust the pH to 5.6 with sodium hydroxide solution, filter through a 0.45μm microporous membrane, and pack in cillin at 5mL / cartridge In the bottle, autoclave 30min obtains the doxofylline injection of embodiment 2.
Embodiment 3
[0045] Dissolve 12.9g of hydroxypropyl-β-cyclodextrin in 100mL of water for injection; use phosphoric acid to adjust the pH=2.3, then add 0.12g of L-serine, stir to dissolve, and obtain hydroxypropyl-β-cyclodextrin containing serine Cyclodextrin aqueous solution; then add 2g doxofylline, stir at 40°C and 400prm for 1.5h, then adjust the pH to 5.2 with sodium hydroxide solution, filter through a 0.45μm microporous membrane, and pack in 5mL / cartridge In the vial, autoclave for 30 minutes to obtain the doxofylline injection of Example 3.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com