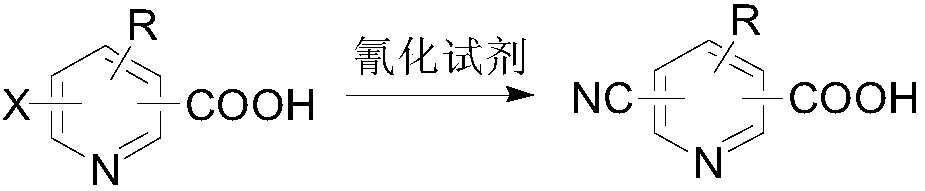
Method for converting halogenopyridinecarboxylic acid into cyanopyridinecarboxylic acid
A technology of halogenated pyridinecarboxylic acid and substituting pyridinecarboxylic acid, which is applied in the field of compound synthesis, can solve problems such as no reports in the literature, and achieve the effects of short process, simplified synthesis steps and broad application prospects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
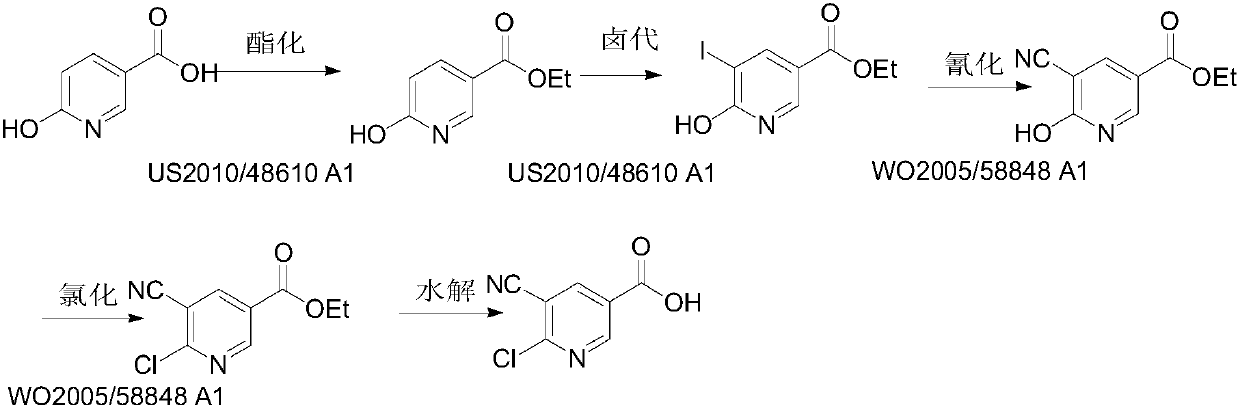
[0032] In this example, 5-cyano-6-hydroxynicotinic acid is synthesized from 5-iodo-6-hydroxynicotinic acid, and the reaction formula is as follows:
[0033]
[0034] Add 50.0g of 5-iodo-6-hydroxynicotinic acid (0.19mol, 1.0eq) and 25g of CuCN (0.28mol, 1.5eq) into 300mL of DMF, heat to an external temperature of 125°C under the protection of nitrogen, keep it for about 5 hours, and the raw materials disappear , stop heating. Remove DMF by rotary steaming, stop the rotary steaming until the viscous porridge in the system does not flow, pour about 500mL MeOH into the bottle, stir well, stir in a low-temperature ice-water bath for about 1 hour, filter, and rinse the filter cake with acetone and PE. The crude product 1B was obtained after drying, and 1B was recrystallized from methanol to obtain 29.1 g of off-white solid 5-cyano-6-hydroxynicotinic acid, with a yield of 93.3%. [M-1] - =163.0, 1 H NMR (400MHz, DMSO) δ 13.16 (brs, 1H), 8.43 (d, J=2.2Hz, 1H), 8.29 (d, J=2.2Hz, 1...
Embodiment 2
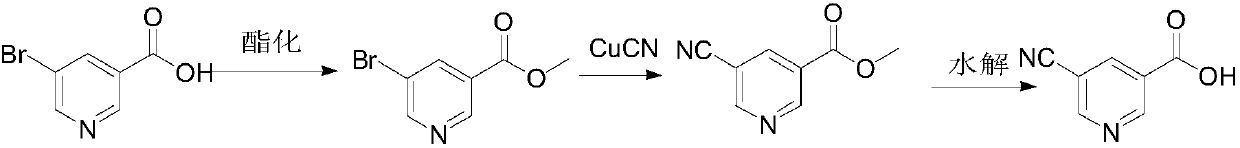
[0036] In this example, 5-cyano-6-hydroxynicotinic acid is synthesized from 5-bromo-6-hydroxynicotinic acid, and the reaction formula is as follows:
[0037]
[0038] Take 5.0g of 5-bromo-6-hydroxynicotinic acid (22.9mmol, 1.0eq) and 4.14g of CuCN (45.9mmol, 2.0eq) into a single-necked bottle containing 40mL of NMP, heat to 155°C under nitrogen protection, and keep warm for 5h , click the plate, the raw material disappears, stop heating. Most of the NMP was removed by rotary evaporation, poured into water, extracted three times with 200 mL of EA, and the organic phase was washed twice with 50 mL of water and 20 mL of saturated sodium chloride respectively. The organic phase was dried and spin-dried to obtain crude product 1B, which was recrystallized from methanol to obtain 3.4 g of off-white solid, yield: 90.5%. [M-1] - =163.0, 1 H NMR (400MHz, DMSO) δ13.16 (br s, 1H), 8.43 (d, J=2.2Hz, 1H), 8.29 (d, J=2.2Hz, 1H).
Embodiment 3
[0040] In this example, 5-cyano-6-hydroxynicotinic acid is synthesized from 5-chloro-6-hydroxynicotinic acid, and the reaction formula is as follows:
[0041]
[0042] Take 4.0g of 5-chloro-6-hydroxynicotinic acid (22.9mmol, 1.0eq) and 5.38g of zinc cyanide (45.9mmol, 2.0eq) into a single-necked bottle containing 40mL of NMP, and heat to 180°C under nitrogen protection. Keep warm for 20 hours, touch the plate, the raw material disappears, stop heating. Most of the NMP was removed by rotary evaporation, poured into water, extracted three times with 200 mL of EA, and the organic phase was washed twice with 50 mL of water and 20 mL of saturated sodium chloride respectively. The organic phase was dried and spin-dried to obtain crude product 1B, which was recrystallized from methanol to obtain 3.1 g of off-white solid, yield: 82.5%. [M-1] - =163.0, 1 H NMR (400MHz, DMSO) δ13.16 (br s, 1H), 8.43 (d, J=2.2Hz, 1H), 8.29 (d, J=2.2Hz, 1H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com