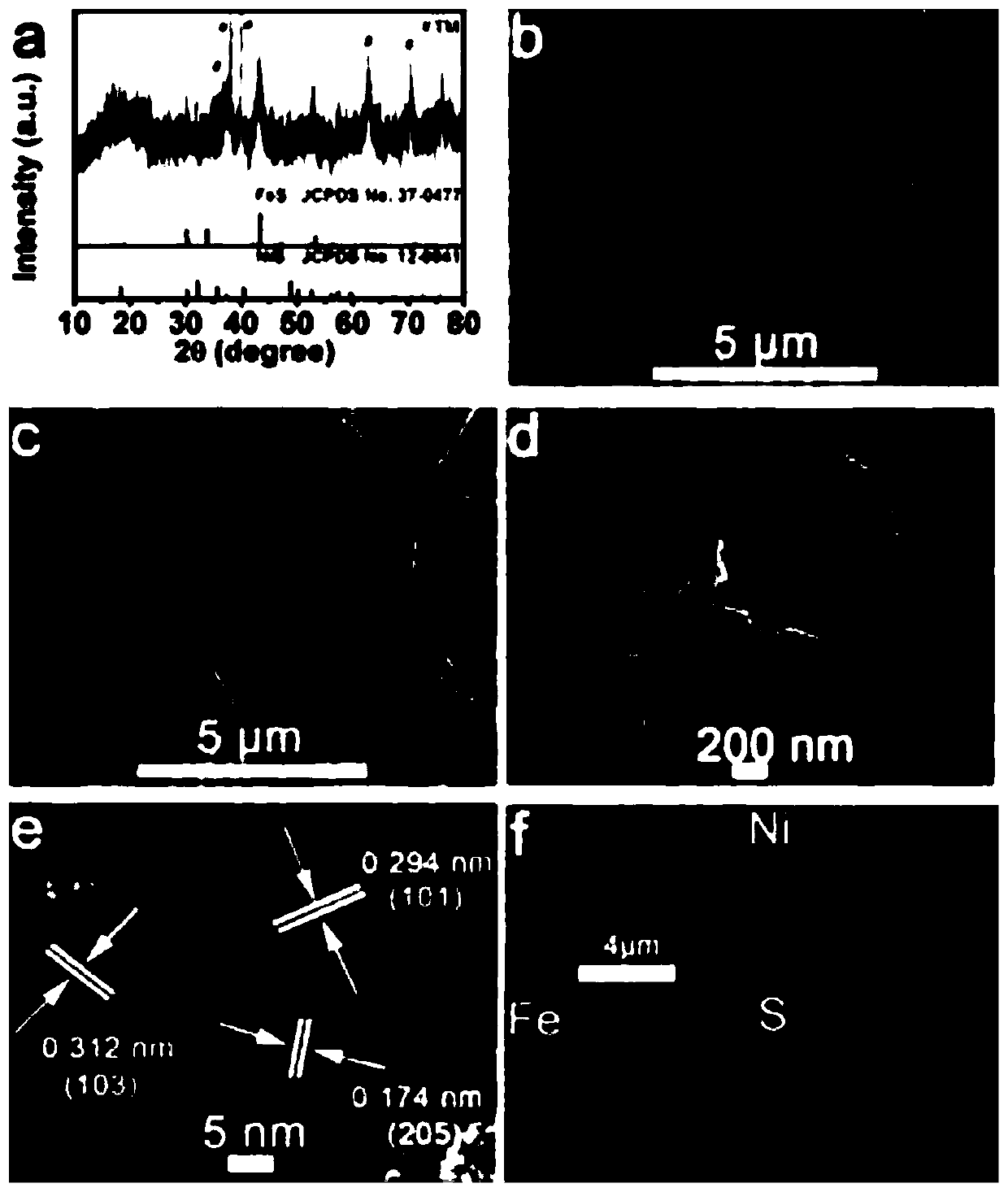
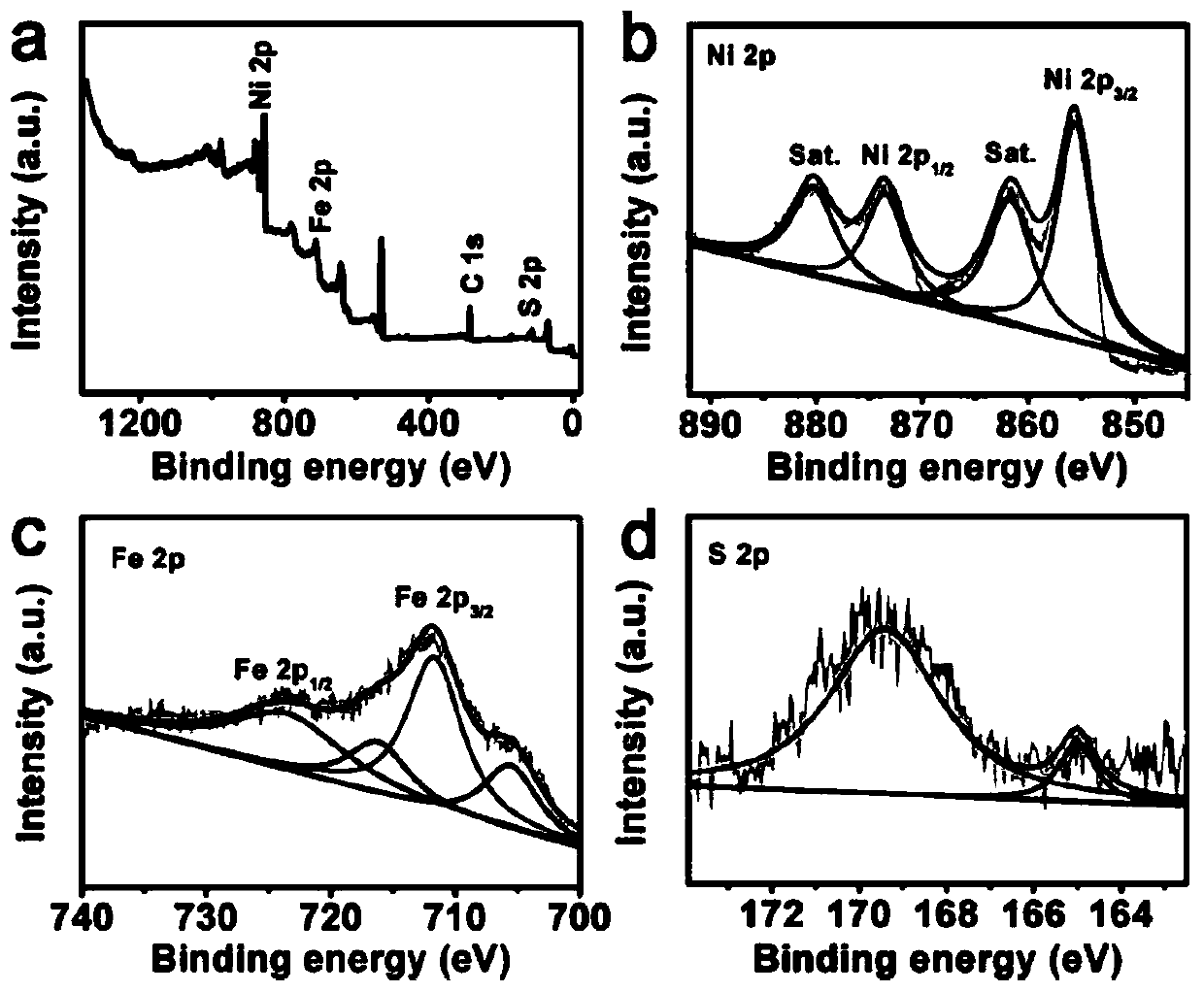
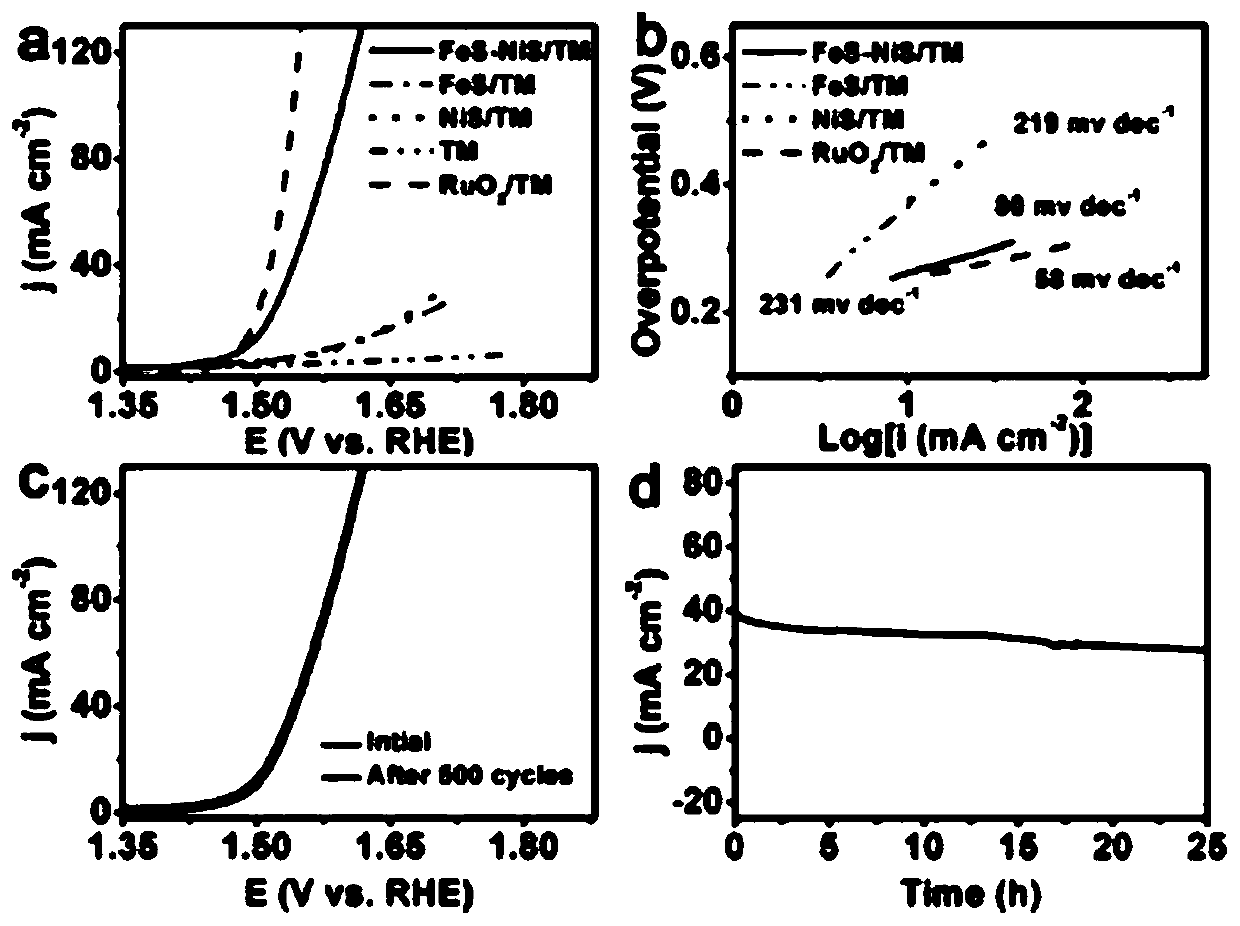
FeS-NiS nano sheet array oxygen evolution catalyst and preparation method and applications thereof
A nanosheet array, catalyst technology, applied in chemical instruments and methods, physical/chemical process catalysts, chemical/physical processes, etc., can solve the problems of high scarcity and cost constraints, and achieve easy materials and high electrochemical conductivity. , the effect of high surface roughness
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] (1) Preparation of NiFe-LDH precursor by hydrothermal method: ① 0.58gNi(NO 3 ) 2 ·6H 2 O, 0.09g Fe(NO 3 ) 3 9H 2 O, 0.60g urea and 0.22g NH 4 F was put into 40mL deionized water and stirred vigorously for 10min to obtain a mixed solution. ② Put the pretreated titanium mesh (2cm×4cm) into ② to prepare a mixed solution and transfer it to a polytetrafluoroethylene autoclave, and keep it warm at 120°C 6h, take it out after cooling to room temperature, rinse with water, and then dry in air at 60°C for 6h to prepare the NiFe-LDH precursor;
[0031] (2) Preparation of FeS-NiS / TM: 1.92g Na 2 Dissolve S in 40 mL deionized water to prepare Na 2 S solution, put the NiFe-LDH precursor prepared in step (1) into Na 2 The S solution was transferred together into a polytetrafluoroethylene autoclave, kept at 120°C for 4h, cooled to room temperature, taken out, rinsed with water, and then dried in air at 60°C for 6h to obtain the finished FeS-NiS / TM product.
Embodiment 2
[0033] (1) Preparation of NiFe-LDH precursor by hydrothermal method: ① 0.6gNi(NO 3 ) 2 ·6H 2 O, 0.15g Fe(NO 3 ) 3 9H 2 O, 0.4g urea and 0.10g NH4 F was put into 40mL deionized water and stirred vigorously for 10min to obtain a mixed solution. ② Put the pretreated titanium mesh (2cm×4cm) into ② to prepare a mixed solution and transfer it to a polytetrafluoroethylene autoclave, and keep it warm at 100°C 8h, take it out after cooling to room temperature, rinse with water, and then dry in air at 45°C for 12h to prepare the NiFe-LDH precursor;
[0034] (2) Preparation of FeS-NiS / TM: 0.3gNa 2 Dissolve S in 40 mL deionized water to prepare Na 2 S solution, put the NiFe-LDH precursor prepared in step (1) into Na 2 The S solution was transferred together into a polytetrafluoroethylene autoclave, kept at 100°C for 8h, cooled to room temperature, taken out, rinsed with water, and then dried in air at 45°C for 12h to obtain the finished FeS-NiS / TM product.
Embodiment 3
[0036] (1) Preparation of NiFe-LDH precursor by hydrothermal method: ① 0.8gNi(NO 3 ) 2 ·6H 2 O, 0.10g Fe(NO 3 ) 3 9H 2 O, 0.5g urea and 0.22g NH 4 F was put into 40mL deionized water and stirred vigorously for 10min to obtain a mixed solution. ② Put the pretreated titanium mesh (2cm×4cm) into ② to prepare a mixed solution and transfer it to a polytetrafluoroethylene autoclave, and keep it warm at 150°C 5h, take it out after cooling to room temperature, rinse with water, and then dry in air at 50°C for 12h to prepare the NiFe-LDH precursor;
[0037] (2) Preparation of FeS-NiS / TM: 0.3gNa 2 Dissolve S in 40 mL deionized water to prepare Na 2 S solution, put the NiFe-LDH precursor prepared in step (1) into Na 2 The S solution was transferred together into a polytetrafluoroethylene autoclave, kept at 150°C for 5h, cooled to room temperature, taken out, rinsed with water, and then dried in air at 50°C for 10h to obtain a finished FeS-NiS / TM product.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com