Making method of human cockayne syndrome specificity adult stem cells
A technology of adult stem cells and syndromes, applied in the biological field, can solve the problems of different genetic backgrounds between humans and model animals, difficulty in obtaining experimental materials, and inability to effectively relieve CS symptoms, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0104] Example 1. Establishment and identification of induced pluripotent stem cell lines carrying CS patient-specific gene mutations
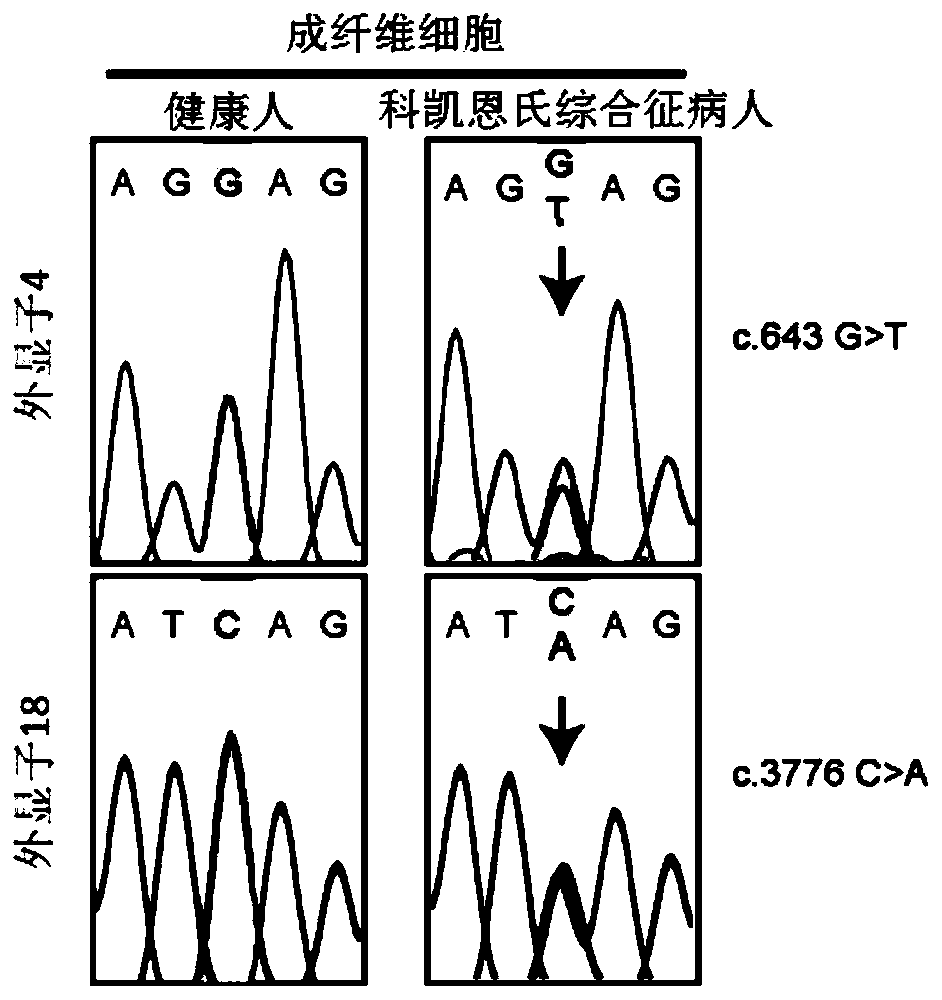
[0105] In order to obtain skin fibroblasts derived from CS patients through primary culture, after the patients signed the informed consent, the present invention carried out ERCC6 heterozygous mutations (c.643G>T, p.E215X (maternal); c.3776C>A, p.S1259X (paternal origin). This mutation has the characteristics of a typical CS pathogenic mutation, and the mutation is a heterozygous nonsense mutation involving the ERCC6 gene, which leads to the premature termination of the synthesis of the ERCC6 peptide chain, which prevents the normal DNA repair in the patient's body. Further leading to the occurrence of CS disease) CS patients undergo diagnostic skin biopsy and retain part of the skin tissue for primary culture to obtain skin fibroblasts. The fragment carrying the mutation was amplified by PCR and DNA sequencing was performed to identify the m...
Embodiment 2
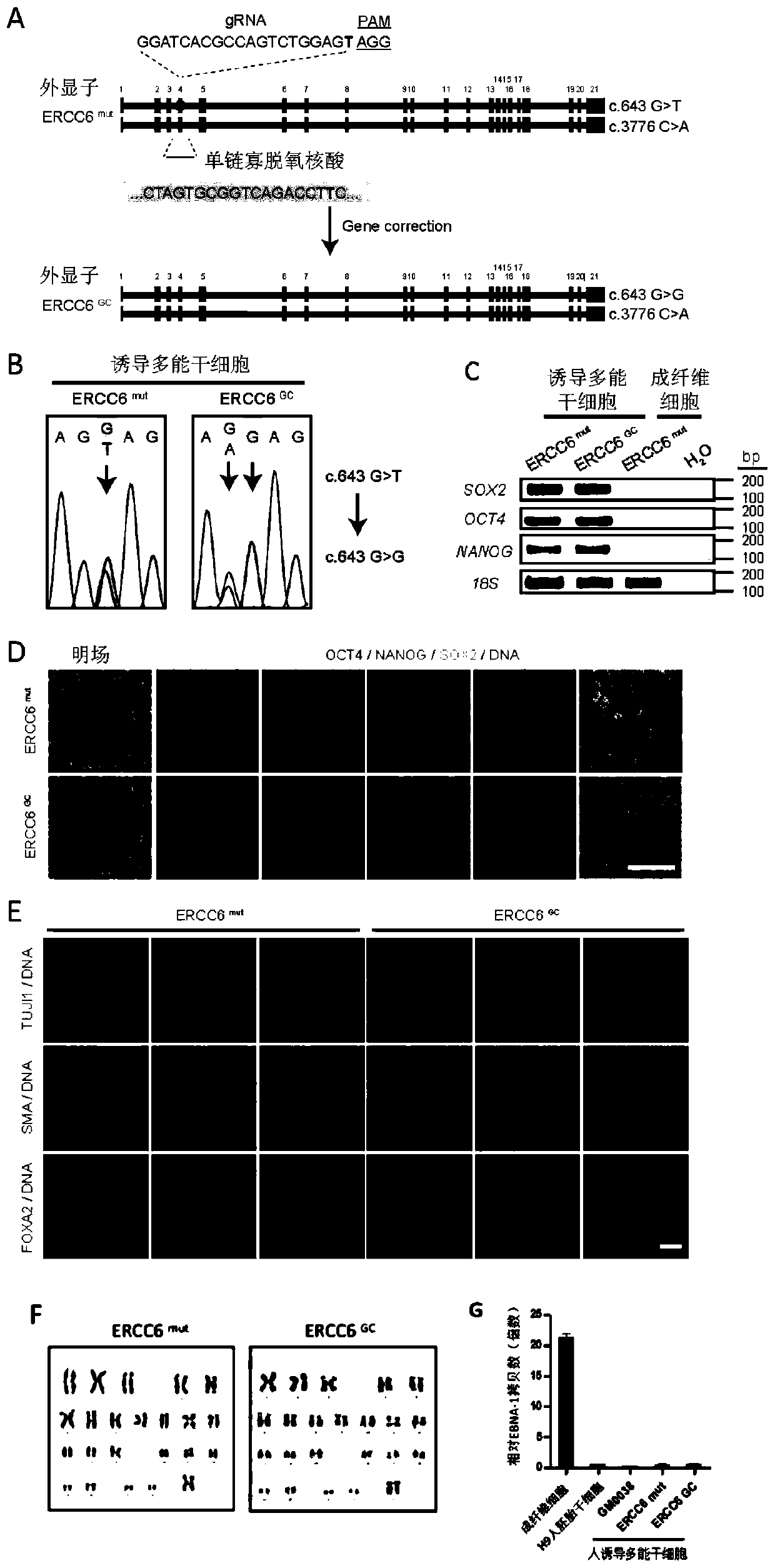
[0122] Example 2. Targeted correction of ERCC6 gene mutations carried by CS-iPSCs
[0123] The gene mutation c.643G>T carried by the iPSC (ERCC6 G643T-iPSC) obtained in Example 1 was targeted to be cleared using CRISPR / Cas9 gene editing technology, and finally the iPSC with the gene mutation c.643G>T eliminated was obtained, and multiple Characterization of competent stem cells.
[0124] Specific steps are as follows:
[0125] 1. Targeted correction of the ERCC6 gene mutation c.643G>T carried by CS-iPSCs
[0126] 1) Cell culture
[0127] The CS-iPSCs obtained in Example 1 were cultured in Matrigel-coated culture plates, and the culture medium was mTESR. When the clone density reaches 70%-80%, digest with TrypLE, count and collect 5×10 6 cells.
[0128] 2) Preparation of electrotransfer solution
[0129] Mix 8 μg of Cas9-GFP plasmid, 8 μg of gene-corrected recombinant template and 4 μg of corresponding gene-corrected gRNA-mCherry plasmid, and dilute to 100 μl with opti-ME...
Embodiment 3
[0167] Example 3. Directed differentiation of CS-specific induced pluripotent stem cells to generate mesenchymal stem cells
[0168] The CS-iPSCs and GC-iPSCs obtained in Examples 1 and 2 were differentiated to obtain CS-MSCs and GC-MSCs by using the stem cell directional induction differentiation technique, the specific method is as follows:
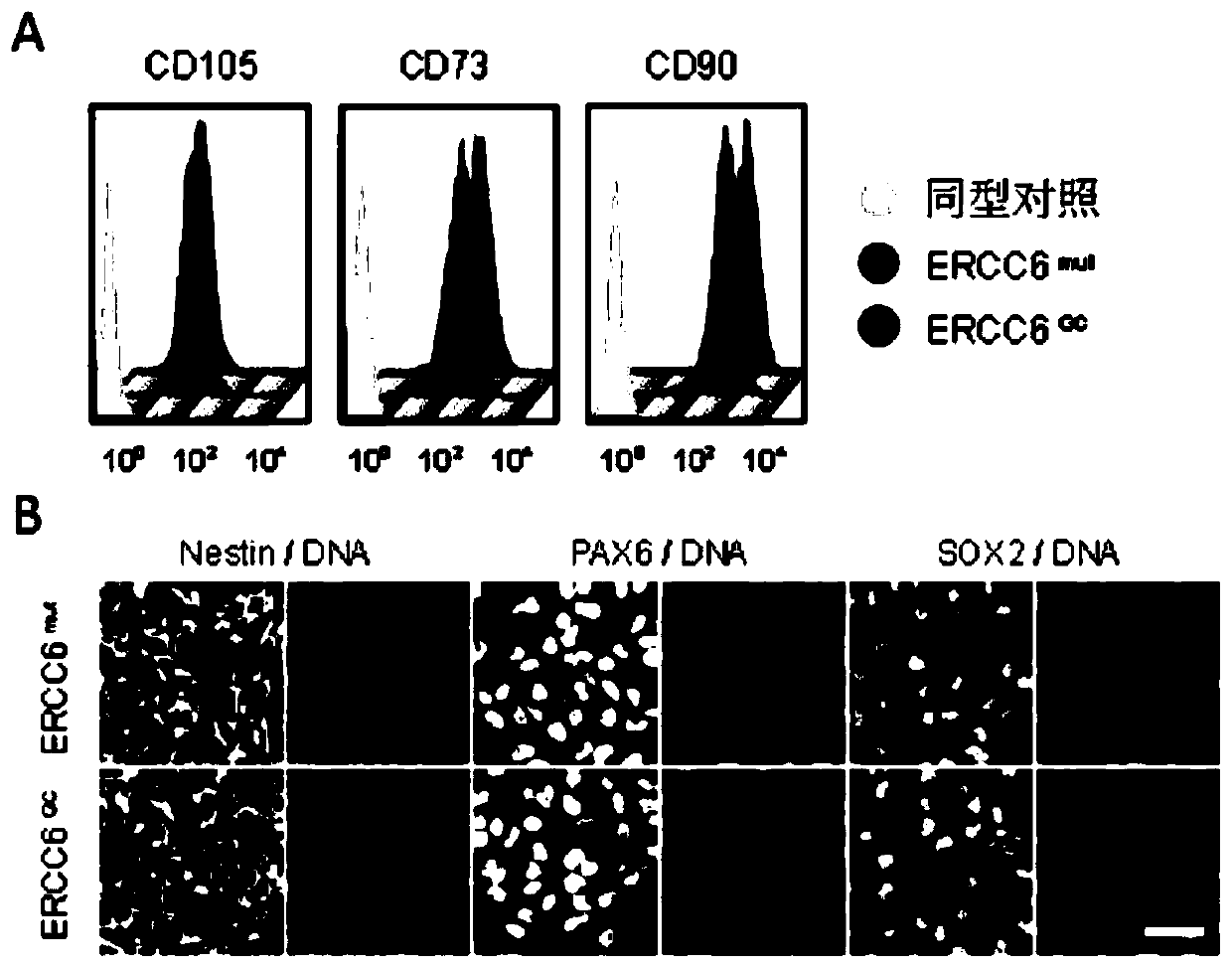
[0169] Method 1: CS-specific and mutation-corrected induced pluripotent stem cells were differentiated into embryoid bodies (EBs) for 3 days, and the EBs were seeded in Matrigel (Invitrogen)-coated 6-well plates with mesenchyme Stem cell medium 1 was used for culturing, and the culture was continued for 2 weeks until fibrous cells appeared. After another passaging, flow cytometry was used to sort the cell populations in which CD73, CD90 and CD105 were all positive (these three proteins are markers of MSC) ( image 3 In A), it is common-grade CS-specific and mutation-corrected mesenchymal stem cells (referred to as CS-MSCs, GC-MSCs).
...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com