Iron-based catalyst and preparation method and application thereof
An iron-based catalyst and catalyst technology, applied in the direction of catalyst activation/preparation, chemical instruments and methods, physical/chemical process catalysts, etc., can solve the problems of high selectivity of low-carbon alkanes, low yield of low-carbon olefins, high selectivity of methane, etc. problems, to achieve the effects of low methane selectivity, high yield of low-carbon olefins, and high catalytic activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] Weigh 16.22g of FeCl 3 ·6H 2 O and 5.96 g FeCl 2 ·6H 2 O was dissolved in 30ml of deionized water, and 2.5ml of concentrated hydrochloric acid was added to form a mixed solution A. Add 200ml of 5% NH to mixed solution A at a flow rate of 3ml / min using a constant flow pump 3 ·H 2 O (solution B), it was placed in a 40°C water bath and stirred continuously, and aged for 0.5h after the dropwise addition was completed. The obtained turbid liquid was filtered and washed until neutral, and then the filter cake was dried in air at 60° C. for 12 h; the dried solid was calcined in air atmosphere at 350° C. for 4 h to obtain solid C.
[0039] Weigh 2.0g of the above solid C, weigh 0.0746g NaNO 3 Dissolve it in 2ml deionized water to make a solution, impregnate the above solid C sample in the above NaNO 3 solution and mix it well. Immerse at room temperature for 12 hours, dry at 60°C for 12 hours, roast at 350°C for 4 hours, and finally grind, tablet, crush, and sieve (20-4...
Embodiment 2
[0043] Weigh the catalyst 1Na / Fe prepared in the method of embodiment 1 3 o 4 CO 2 Hydrogenation reaction performance evaluation.
[0044] Catalyst reduction conditions: the reducing gas is pure H 2 , purity>99.9%, space velocity is 1800h -1 , the heating rate is 5°C / min, the reduction temperature is 350°C, the pressure is normal pressure, and the reduction time is 12h.
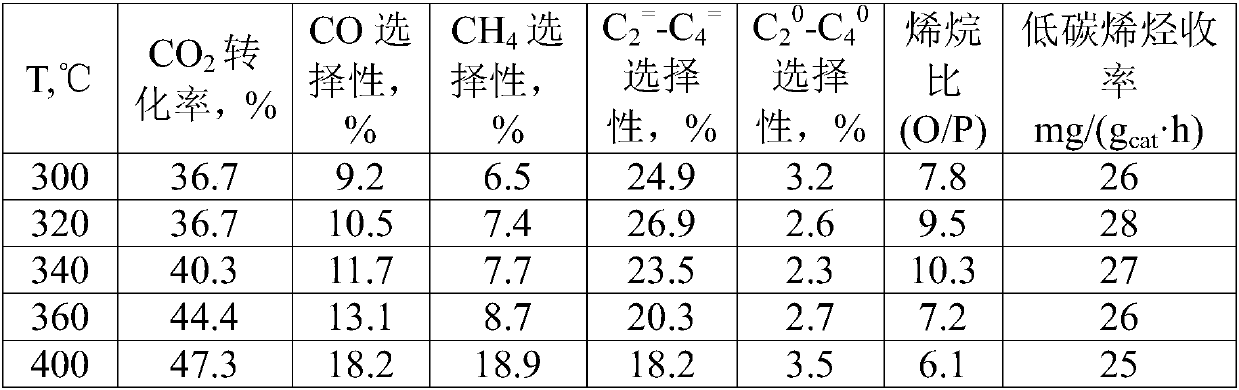
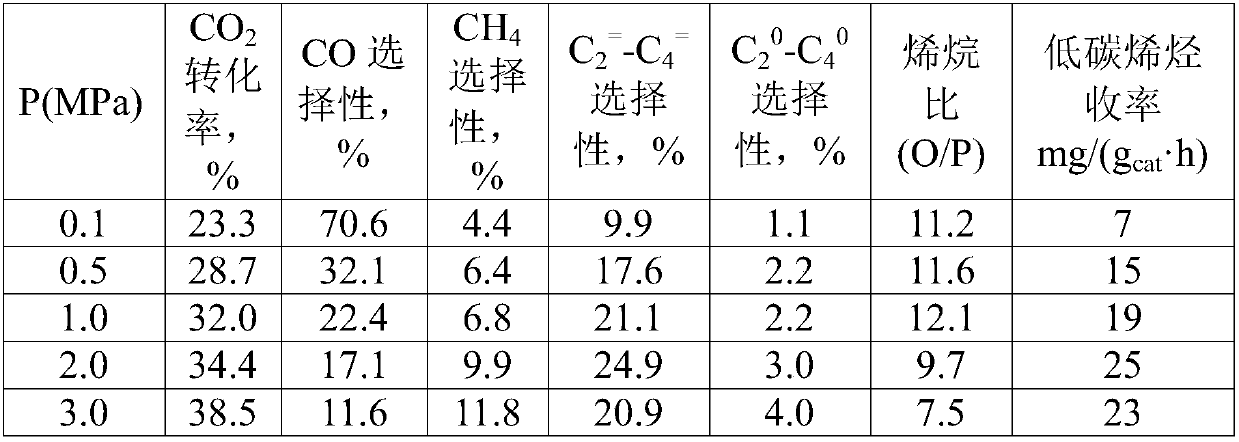
[0045] CO 2 Reaction conditions for the reaction of hydrogenation to synthesize light olefins: molar ratio H 2 :CO 2 =3, the space velocity is 2000mL / (g cat h), the temperature was 320°C, and the influence of the reaction pressure on the performance of the catalyst was investigated. The results are shown in Table 2. From the data analysis, it can be seen that as the pressure increases, the CO 2 The conversion rate increases gradually, low carbon olefins (C 2 = -C 4 = ) and the olefin ratio (O / P) first increased and then decreased, and the optimal pressure was 2MPa.
Embodiment 3
[0047] Weigh the catalyst 1Na / Fe prepared in the method of embodiment 1 3 o 4 CO 2 Hydrogenation reaction performance evaluation.
[0048] Catalyst reduction conditions: the reducing gas is pure H 2 , purity>99.9%, space velocity is 1800h -1 , the heating rate is 5°C / min, the reduction temperature is 350°C, the pressure is normal pressure, and the reduction time is 12h.
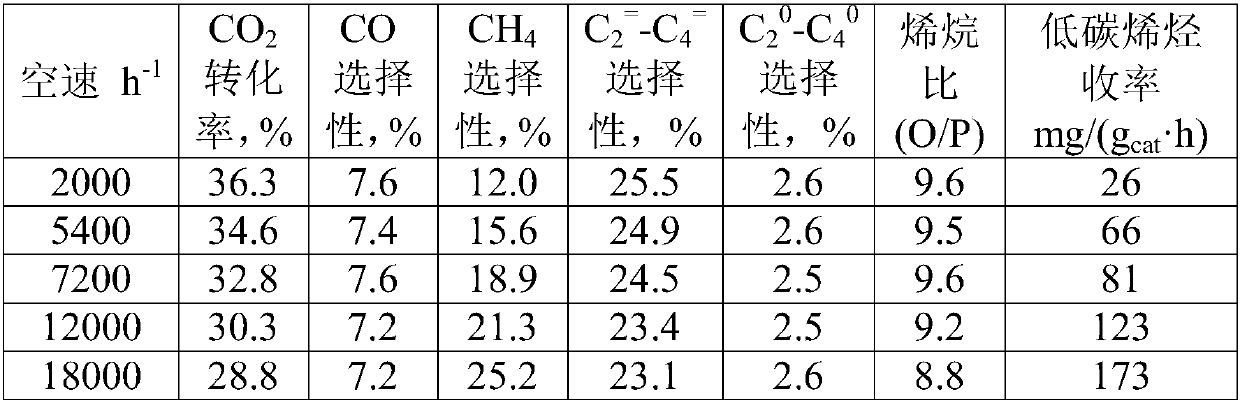
[0049] CO 2 Reaction conditions for the reaction of hydrogenation to synthesize light olefins: molar ratio H 2 :CO 2 =3, the pressure is 2MPa, the temperature is 320°C, and the influence of the reaction space velocity on the catalyst performance is investigated, and the results are shown in Table 3. From the data analysis, it can be seen that with the increase of space velocity, the CO 2 The conversion rate decreases gradually, and the low carbon olefins (C 2 = -C 4 = ) The selectivity and olefin ratio (O / P) change little, and a higher yield of low-carbon olefins can be obtained at high space velo...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com