MnO2-NiO active coke low-temperature denitration catalyst and preparation method thereof
A technology for low-temperature denitration and activated coke, which is applied in chemical instruments and methods, physical/chemical process catalysts, metal/metal oxide/metal hydroxide catalysts, etc. Low, wide range of carrier sources, environment-friendly effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
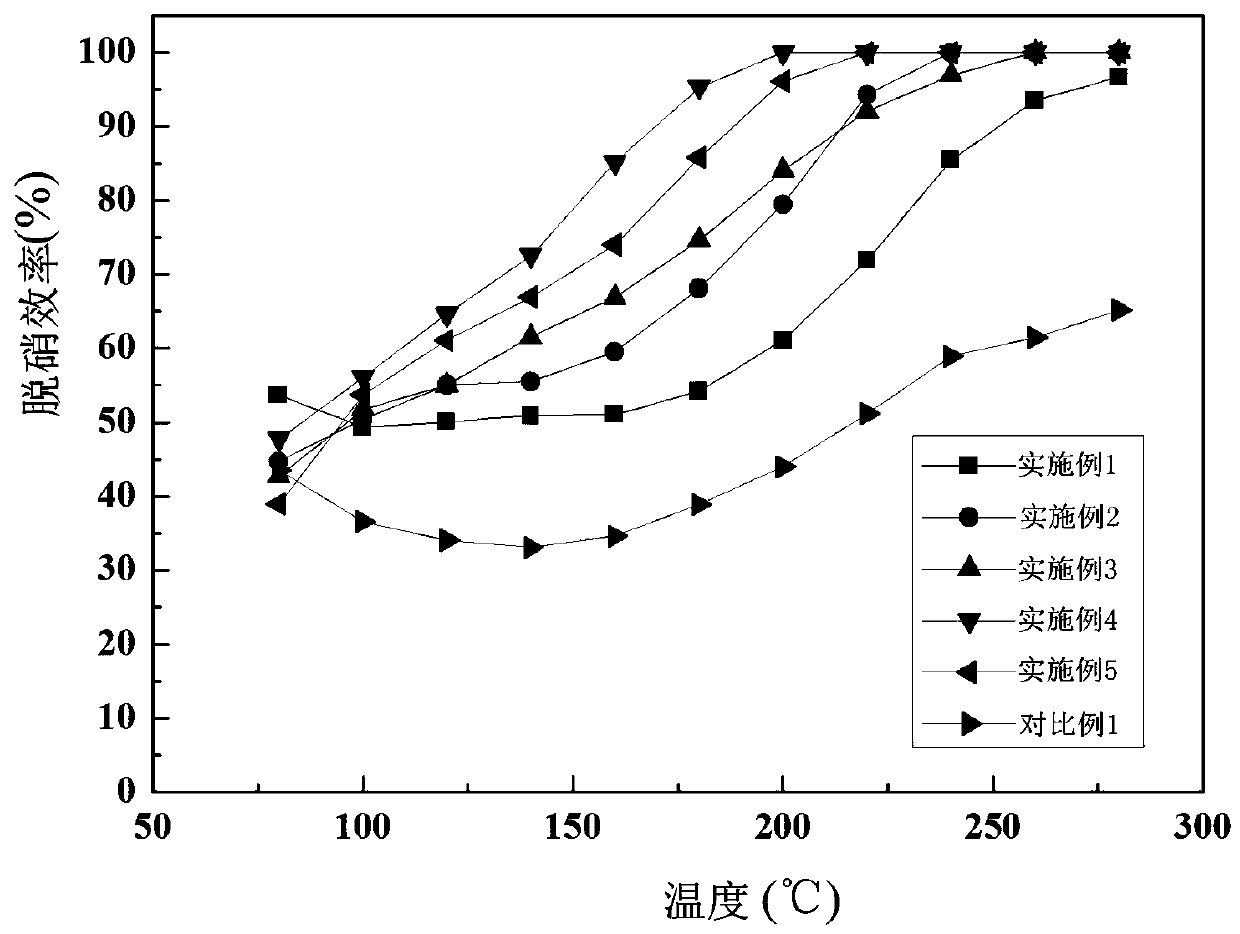
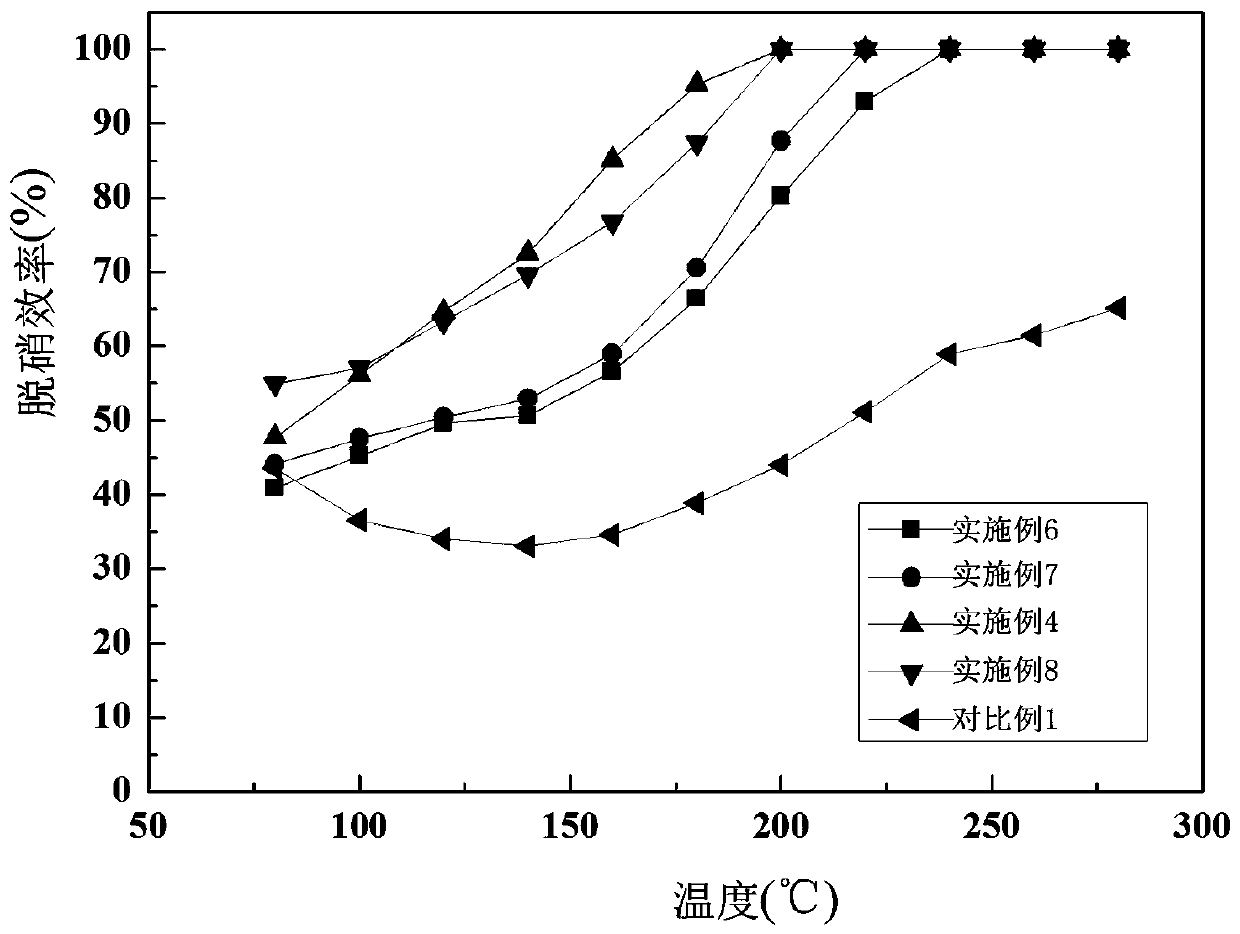
Embodiment 1
[0029] Screen active coke (AC) particles 30-80 mesh, wash with deionized water to pH about 7, then place in an oven to bake for 2 hours, soak the dried active coke in a 5mol / L nitric acid solution in a water bath at 80℃ Stir at medium temperature for 2 hours, then wash with deionized water to a pH of about 7, and then bake at 110°C for 12 hours. Weigh 0.695g of 50% manganese nitrate (Mn(NO3)2) solution and 0.56g of nickel nitrate hexahydrate (Ni(NO3)2·6H2O) in 7.5mL of deionized water, stir well to obtain a mixed solution; weigh 6g for activation The latter activated coke is immersed in the mixed solution in equal volume, first stirred at room temperature for 2 hours, and then allowed to stand at room temperature for 24 hours, the loading of manganese and nickel is 5%, then dried overnight at 50°C, and then baked at 110°C for 5 hours . The dried sample was calcined in a muffle furnace under a nitrogen atmosphere at 400°C for 2h, with a heating rate of 5°C / min, and a nitrogen f...
Embodiment 2
[0031] Screen active coke particles 30-80 mesh, wash with deionized water until the pH is about 7, then place in an oven to bake for 2 hours, soak the dried active coke in a 5mol / L nitric acid solution, and stir in a water bath at 80°C for 2 hours , And then washed with deionized water to a pH of about 7, and then bake at 110 ℃ for 12 hours. Weigh 2.46g of 50% manganese nitrate (Mn(NO3)2) solution and 1.99g of nickel nitrate hexahydrate (Ni(NO3)2·6H2O), dissolve in 12mL of deionized water, stir evenly to obtain a mixed solution; weigh 10g after activation The activated coke is immersed in the mixed solution in equal volume, first stirred at room temperature for 2h, and then allowed to stand at room temperature for 24h, the loading of manganese and nickel is 10%, then dried overnight at 50℃, and then baked at 110℃ for 5h. The dried sample was calcined in a muffle furnace under a nitrogen atmosphere at 400℃ for 2h, with a heating rate of 5℃ / min, and a nitrogen flow rate of 200mL / ...
Embodiment 3
[0033] Screen active coke particles 30-80 mesh, wash with deionized water until the pH is about 7, then place in an oven to bake for 2 hours, soak the dried active coke in a 5mol / L nitric acid solution, and stir in a water bath at 80°C for 2 hours , And then washed with deionized water to a pH of about 7, and then bake at 110 ℃ for 12 hours. Weigh 5.53g of 50% manganese nitrate (Mn(NO3)2) solution and 4.5g of nickel nitrate hexahydrate (Ni(NO3)2·6H2O), dissolve in 12mL of deionized water, stir evenly to obtain a mixed solution; weigh 10g after activation The activated coke is immersed in the mixed solution in equal volume, first stirred at room temperature for 2 hours, and then allowed to stand at room temperature for 24 hours, the loading of manganese and nickel is 15%, then dried overnight at 50°C, and then baked at 110°C for 5 hours. The dried sample was calcined in a muffle furnace under a nitrogen atmosphere at 400℃ for 2h, with a heating rate of 5℃ / min, and a nitrogen flo...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size (mesh) | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com