Serum-free suspension system for lentiviral production
A production system and lentivirus technology, which are applied in the field of serum-free suspension systems for lentivirus production, and can solve problems such as increasing costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0176] Example 1. Cell Culture
[0177] Suspend cells in LV on an orbital shaker at 30% to 40% of the shake flask size volume in polycarbonate, disposable sterile Vent-up shake flasks (125 mL to 1 L) at 125 rpm in a cell culture incubator (37 °C, 8% CO2, 70% to 80% humidity). Cells were isolated every 3 to 4 days at a density of 4 to 5 x 10 6 / mL.
example 2
[0178] Example 2. Lentivirus production experiment
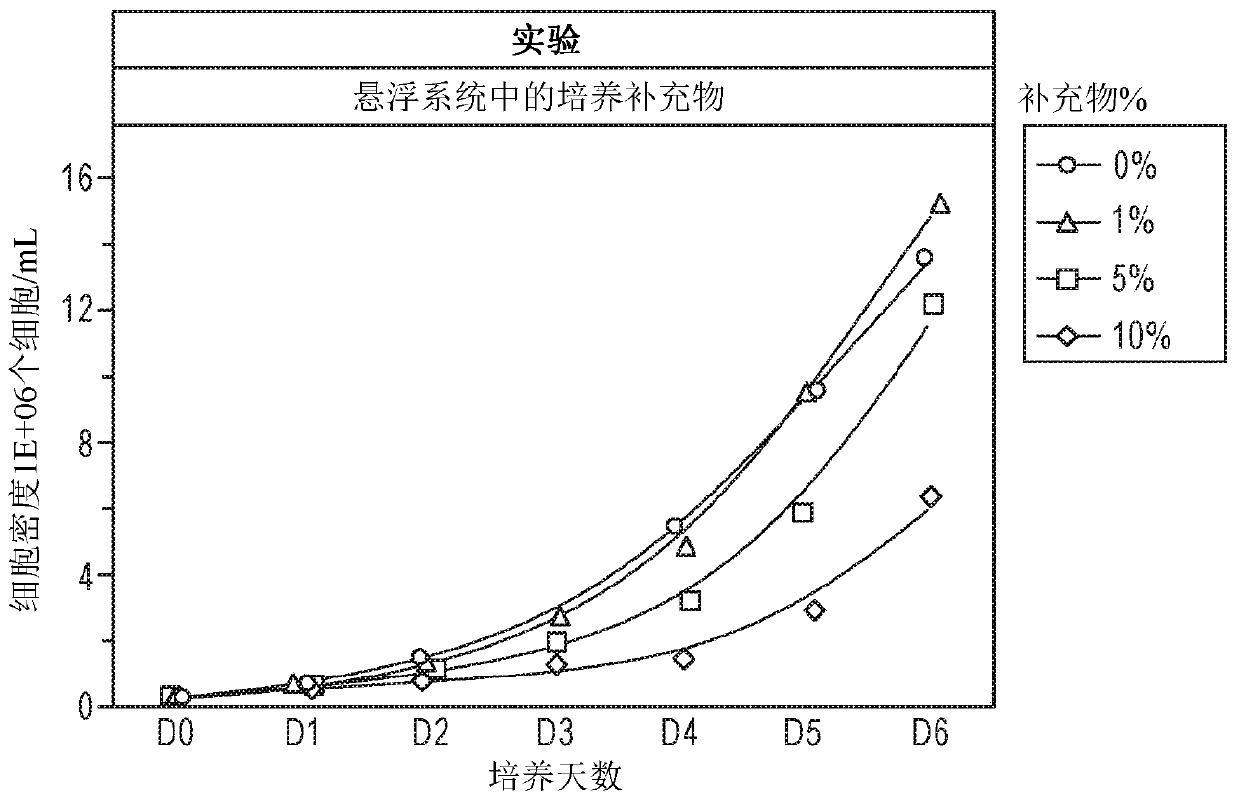
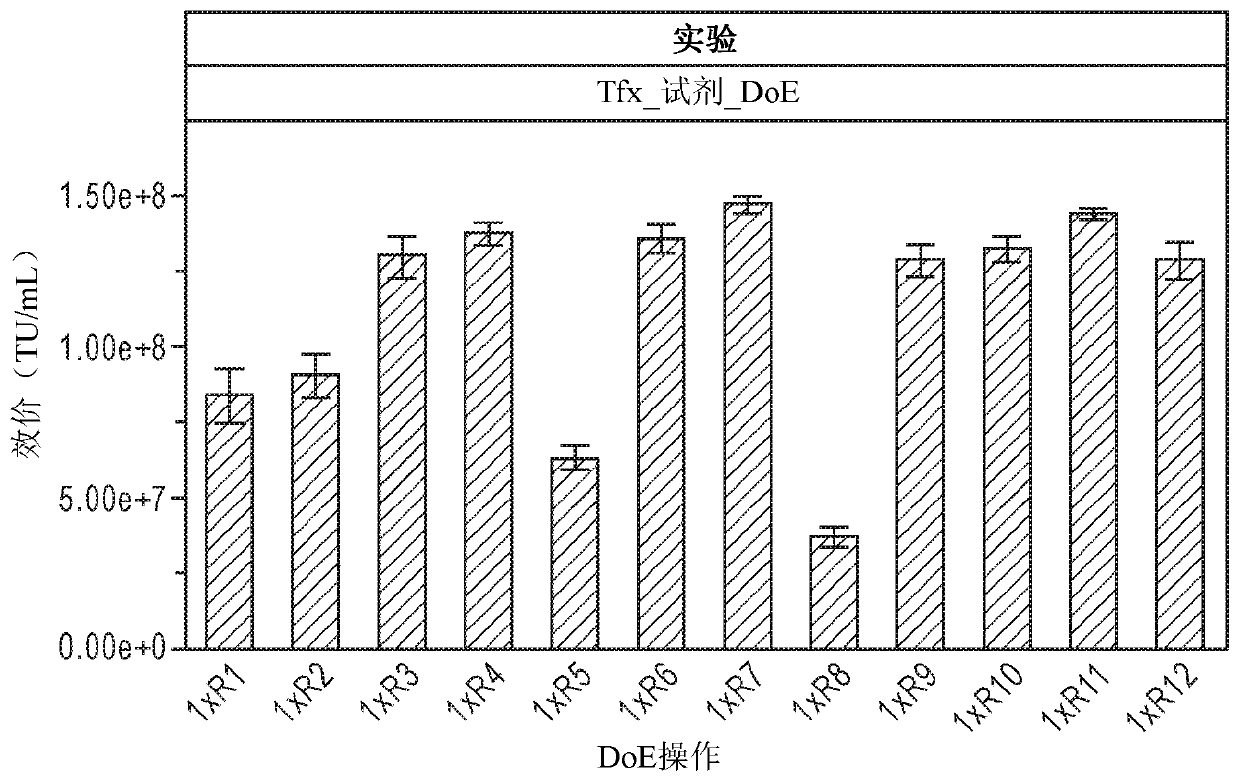
[0179] Based on development needs, lentivirus (LV) production experiments were performed in two formats: 96-well deep-well plates and 125 mL shake flasks, with 1 mL and 30 mL culture volumes, respectively. The cell density was 4 x 10 6 / mL. Lentiviral vectors (LVV) were packaged by co-transfecting lentiviral expression (transfer) plasmid-pLenti6.3 / V5-GW / EmGFP and lentiviral packaging plasmid-ViraPower lentiviral packaging mixture with a total DNA of 3 μg / mL. For both transfection reagent and DNA diluent, Opti- I is the DNA / reagent complex medium at 75uL / mL; the total complex volume is 150ul / mL. The complexation time is 10 minutes to 20 minutes. ExpifectamineTM 293 is a transfection reagent developed by LV culture supplement and LV enhancer, the dosage is 5ul / mL to 8ul / mL. 5% LV Culture Supplement is included in all assays under development. Prior to the development described here, 5 mM caffeine was used as an LV enha...
example 3
[0180] Example 3: Lentivirus titer measurement
[0181] Lentivirus titers were measured by infecting Ht1080 cells. Four hours before infection, 7000 cells per well were seeded in 96-well plates. Upon infection, cells were attached to the culture vessel at approximately 30% confluency. Containing 8ug / mL in the culture medium for 10 1 to 10 5 Serial dilutions of LVV. Pass 10 4 and 10 5 Virus dilutions infect cells. After infecting the cells, the infected Ht1080 plates were spun at 2000 rpm for 30 minutes at room temperature. 18 hours after infection, use without Replace the medium with fresh medium. Cells were incubated for an additional 72 hours. And GFP positive cells were measured by Attune flow cytometry. Titers were calculated based on the percentage of GFP cells between 1% and 20% of wells.
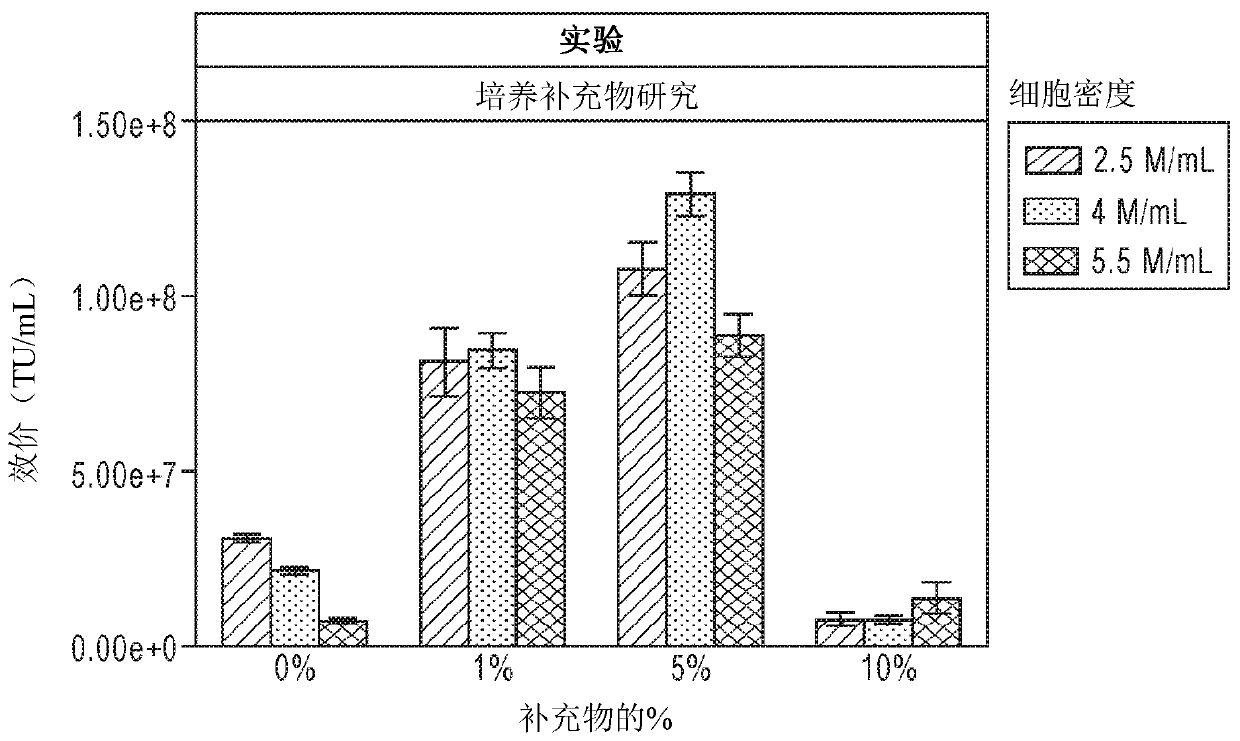
[0182] On the day of transfection, prepare 30 mL cultures of 3 different cell densities in 125 mL shake flasks: 2.5 x 10 6 / mL, 4×10 6 / mL and 5×10 6 / mL. Each dens...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com