Renewable polyurea-urethane based on dynamic covalent crosslinking of stable borate bonds, and preparation method and application thereof
A covalent cross-linking and borate bond technology, applied in polyurea/polyurethane coatings, polyurea/polyurethane adhesives, chemical instruments and methods, etc., can solve the problems of difficult to obtain strong materials and easy damage, etc. Achieve the effect of reducing complexity, increasing productivity, and excellent mechanical properties
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0060] A kind of renewable polyurea-urethane (PUU) preparation method based on stable borate bond of the present invention comprises the following steps: according to the molar ratio of active hydrogen and isocyanate group is 1: (0.8~1.5), nitrogen boron Dissolve the internally coordinated cyclic borate compound (abbreviated as CBC) and isocyanate compound in an organic solvent, stir well until a uniform solution is formed, and react at 40-90°C for 2-10 hours without using a catalyst to obtain PUU resin solution. This method can be called a one-step preparation method.
[0061] Another kind of renewable polyurea-urethane (PUU) preparation method based on stable borate bond of the present invention, comprises the following steps (1), according to the molar ratio of active hydrogen and isocyanate group is (1.2~2.5): 1 , Weigh the CBC and the isocyanate compound and dissolve them in an organic solvent respectively to obtain A solution and B solution. At 40-70°C, slowly add solu...
Embodiment 1
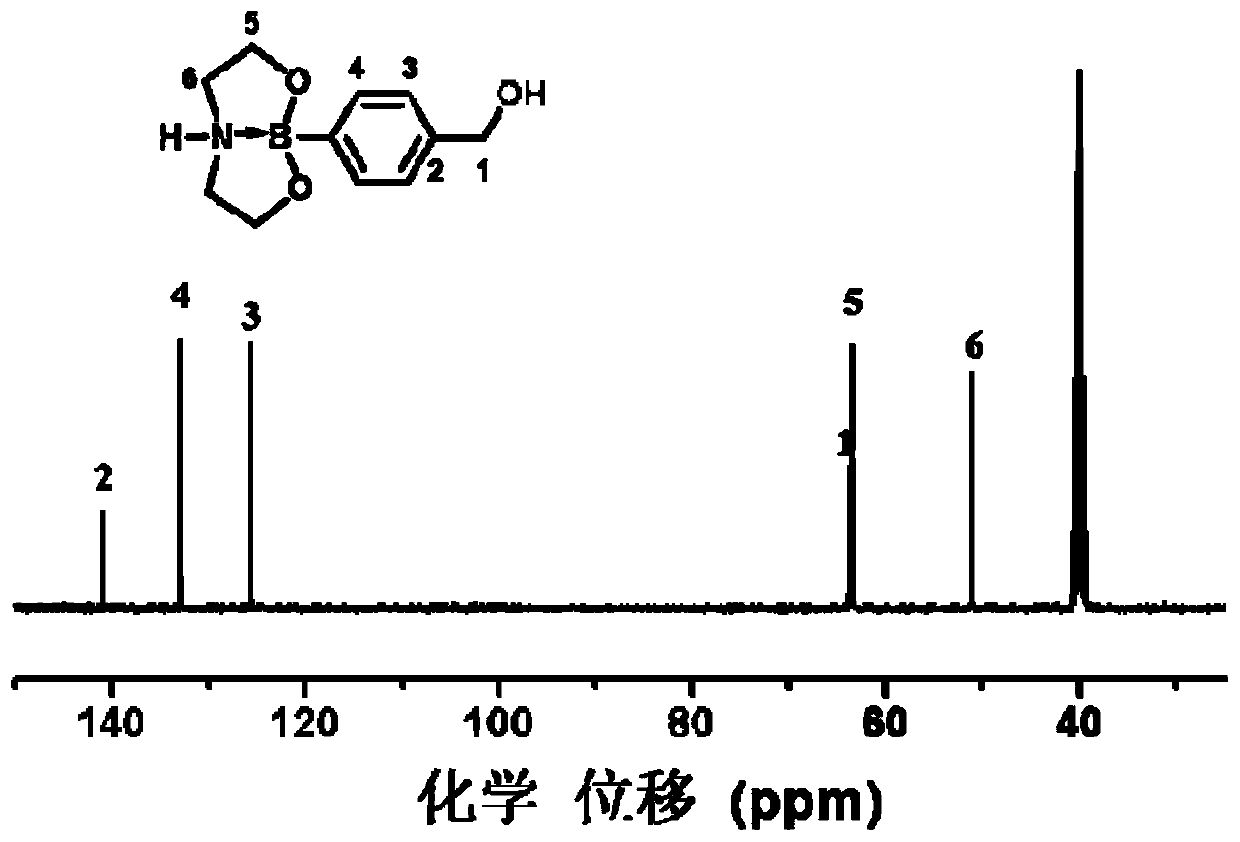
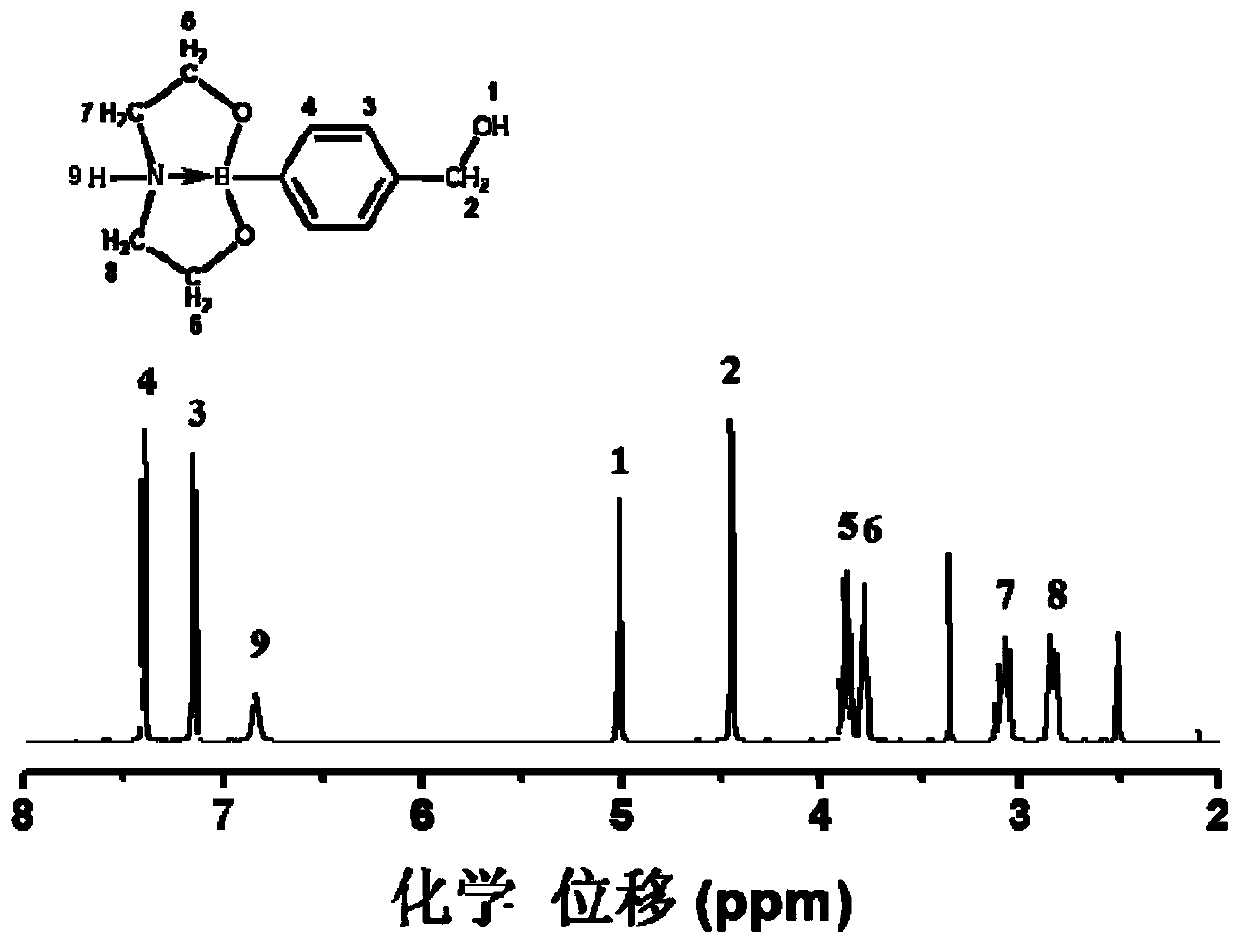
[0079] Synthesis of CBC: Measure 200mL of distilled water into a 500mL beaker, add 15g of hydroxymethylphenylboronic acid and 11g of diethanolamine under magnetic stirring, stir at 50°C until the solution is clear, and keep stirring for 30min. After most of the water was distilled off by a rotary evaporator (temperature 90°C, vacuum degree 0.06MPa), the crude product was obtained by suction filtration with a Buchner funnel and rinsed with IPA several times, and the above crude product was added to a certain amount of DMF. , heated to 100°C to make it fully dissolved, slowly lowered to room temperature, cooled in an ice-water bath for 2h, and then filtered with a Buchner funnel, the resulting product was washed with acetone for 3 times, and vacuum-dried at 80°C for 3h to obtain a white crystal product. The rate is 88%.
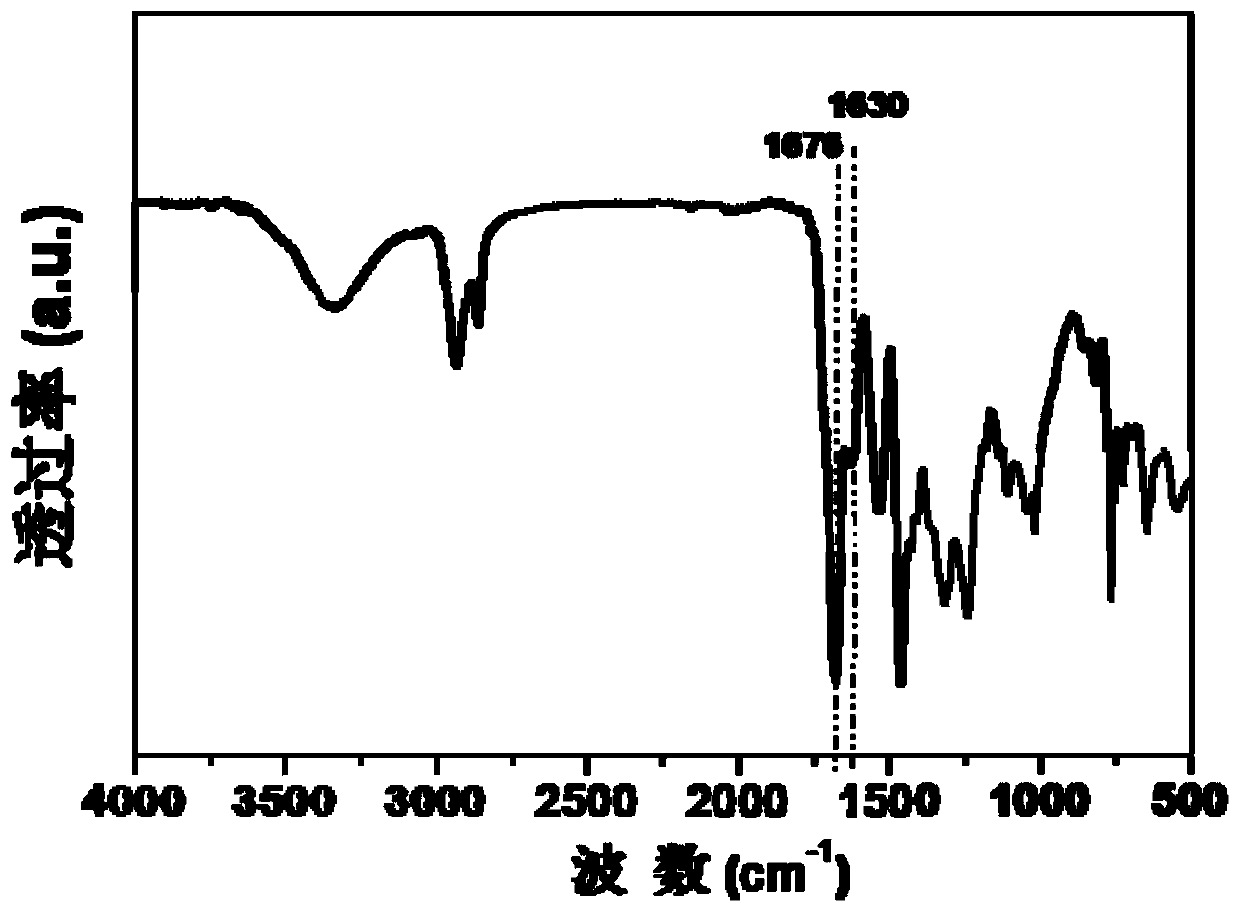
[0080] Synthesis of PUU: In a three-necked flask equipped with a stirrer, a thermometer and a condensation device, 9g CBC, 20g HDI triisocyanate (that is, the ...
Embodiment 2
[0089] Synthesis of CBC: Measure 200mL of distilled water into a 500mL beaker, add 20g of p-aminophenylboronic acid and 15g of triethanolamine under magnetic stirring, stir at 60°C until the solution is clear, and keep stirring for 30min. After most of the water was distilled off by a rotary evaporator (temperature 90°C, vacuum degree 0.06MPa), the crude product was obtained by suction filtration with a Buchner funnel and rinsed with IPA several times, and the above crude product was added to a certain amount of DMF. , heated to 100°C to make it fully dissolved, slowly lowered to room temperature, cooled in an ice-water bath for 1.5h, and then filtered with a Buchner funnel. The resulting product was washed with acetone for 3 times, and vacuum-dried at 80°C for 2h to obtain a white crystal product. The yield was 90%.
[0090] Synthesis of PUU: In a three-necked flask equipped with a stirrer, a thermometer and a condensation device, under a nitrogen atmosphere, 21g CBC, 25g TDI...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Glass transition temperature | aaaaa | aaaaa |
| Tensile strength | aaaaa | aaaaa |
| Young's modulus | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com