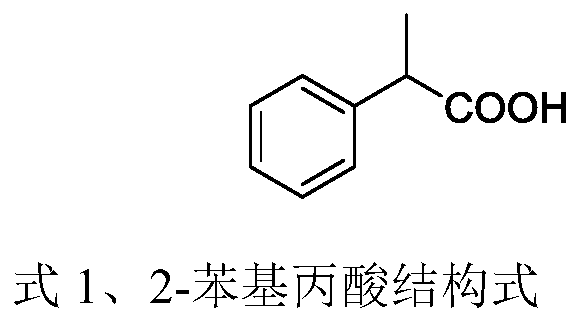
High-pressure synthesis method of 2-phenylpropionic acid
A technology for phenylpropionic acid, high pressure synthesis, applied in chemical instruments and methods, preparation of organic compounds, preparation of carboxylate and other directions, can solve the problems of low production efficiency, large consumption of raw materials, slow reaction speed, etc., to improve production Efficiency, less raw material consumption, and faster reaction rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
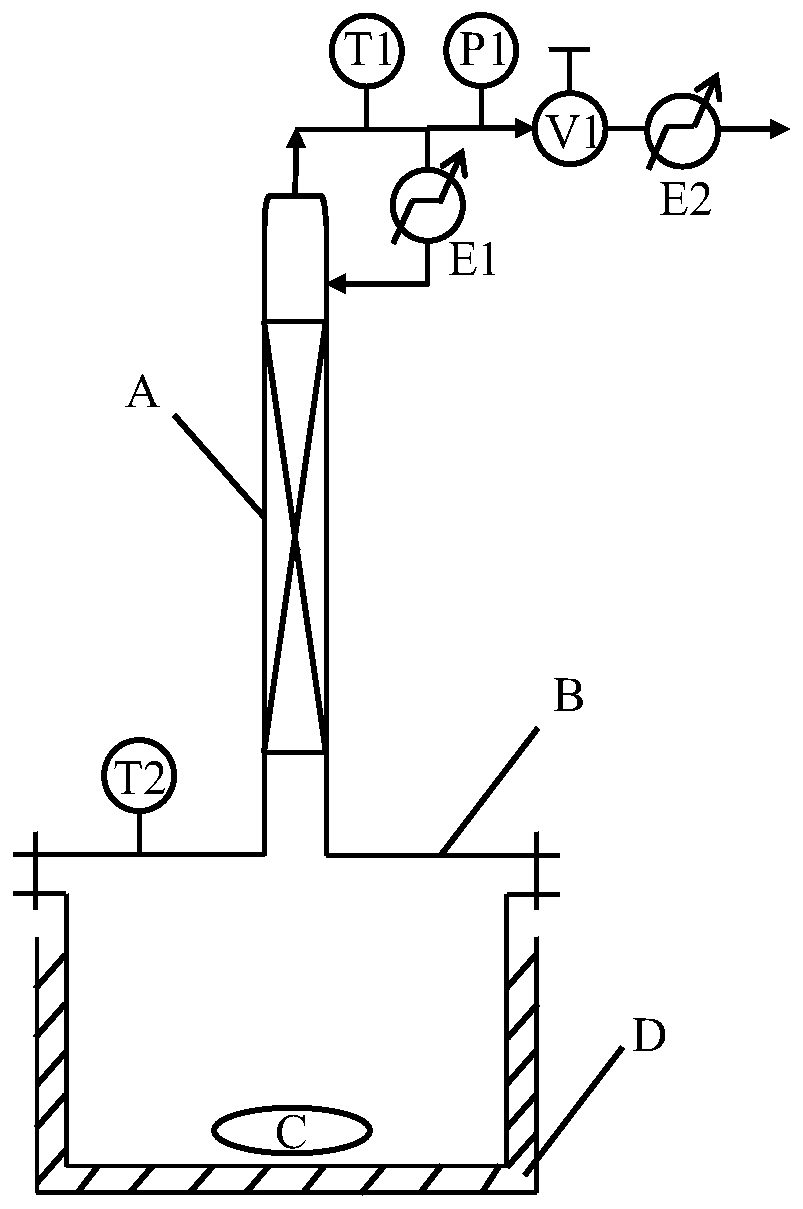
[0046] Example 1, a pressurized reaction-rectification device, such as figure 1 Said, including reactor B (column) with magnetic stirrer C and reaction temperature sensor T2, heating jacket D is set on the outer surface of reactor B, also includes rectifying column A, condenser E1, cooler E2, exhaust pressure control valve V1, pressure sensor P1, distillate temperature sensor T1;
[0047] The top outlet of reaction kettle B is connected with rectification tower A; the top outlet of rectification tower A is connected with cooler E2, and the fraction temperature sensor T1, pressure Sensor P1, exhaust pressure control valve V1; a condenser E1 is set between the side wall outlet near the top of the rectification tower A and the pressure sensor P1.
[0048] The heating jacket D is responsible for heating the reactor B, thereby controlling the reaction temperature in the reactor B; the reaction temperature sensor T2 is used to monitor the reaction temperature in the reactor B; the ...
Embodiment 1
[0050] Embodiment 1, a kind of method for synthesizing 2-phenylpropionic acid by autoclave method, carries out following steps successively:
[0051] Step 1), methylation reaction:
[0052]Add 15g (0.128mol) phenylacetonitrile, 7.5g (0.141mol) sodium methylate, 20.7g (0.23mol) dimethyl carbonate in the reaction kettle B of the pressurized reaction-rectification device, nitrogen leak detection and replacement, then heat up To 180°C (that is, T2 is 180°C), through the exhaust pressure control valve V1, control the top pressure of rectification tower A to 2Mpa (that is, P1 is 2MPa); the reaction time is 4h;
[0053] Gases emitted during the methylation reaction (including dimethyl carbonate, methanol, CO 2 ) enters the rectification tower A through the outlet at the top of the reactor B, and the top temperature of the rectification tower A is about 145°C (that is, T1 is 145°C); the methanol and carbon dioxide in the gas come out from the top of the rectification tower A , after...
Embodiment 2~9
[0063] Change the mol ratio of phenylacetonitrile, sodium methylate, dimethyl carbonate in step 1) in embodiment 1, conditions such as temperature of reaction, reaction time, all the other operations are equal to embodiment 1, obtain embodiment 2~9, concrete process parameter and The comparison between the results and Example 1 is shown in Table 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com