Benzothiazole compounds and application thereof in preparation of bacterial biofilm inhibitors
A technology of bacterial biofilm and benzothiazole, which is applied in the field of medicine, achieves the effects of good induction activity, high safety, and small toxic and side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
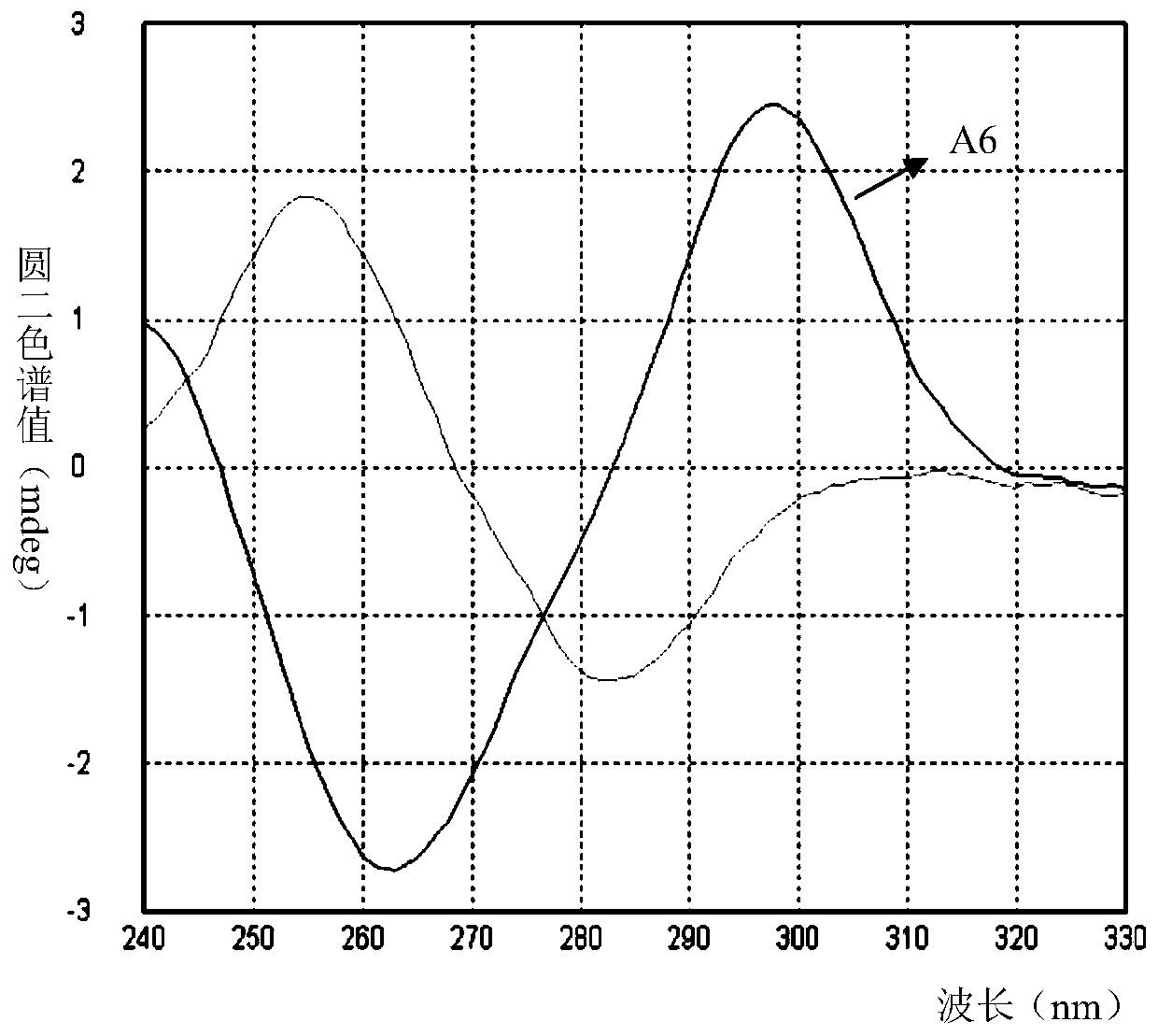
Image
Examples
Embodiment 1
[0050] Example 1: Synthesis of 2,3-dimethylbenzo[d]thiazole-3-iodide (synthesis of compound 2)
[0051] Dissolve 0.76ml (6mmol) of 2-methylbenzothiazole in 10ml of acetonitrile solution, add 0.94ml (15mmol) of methyl iodide, and heat the mixture to reflux for 24h under a nitrogen atmosphere, cool to room temperature, filter with suction, and use a small amount of acetonitrile and After washing with ether, 1.42 g of white solid was obtained. The yield was 83%. 1 H NMR (400 MHz, DMSO) δ8.49(d, J=8.1Hz, 1H), 8.28(d, J=8.4Hz, 1H), 7.85(t, J=7.8 Hz, 1H), 7.77(t, J=7.7Hz, 1H), 4.23(s, 3H), 3.23(s, 3H); 13 C NMR(101MHz, DMSO)δ177.45,141.98,129.70,129.07,128.45,125.02,117.26,37.29,18.40. ESI-MS(m / z):164[M-I] + .
Embodiment 2
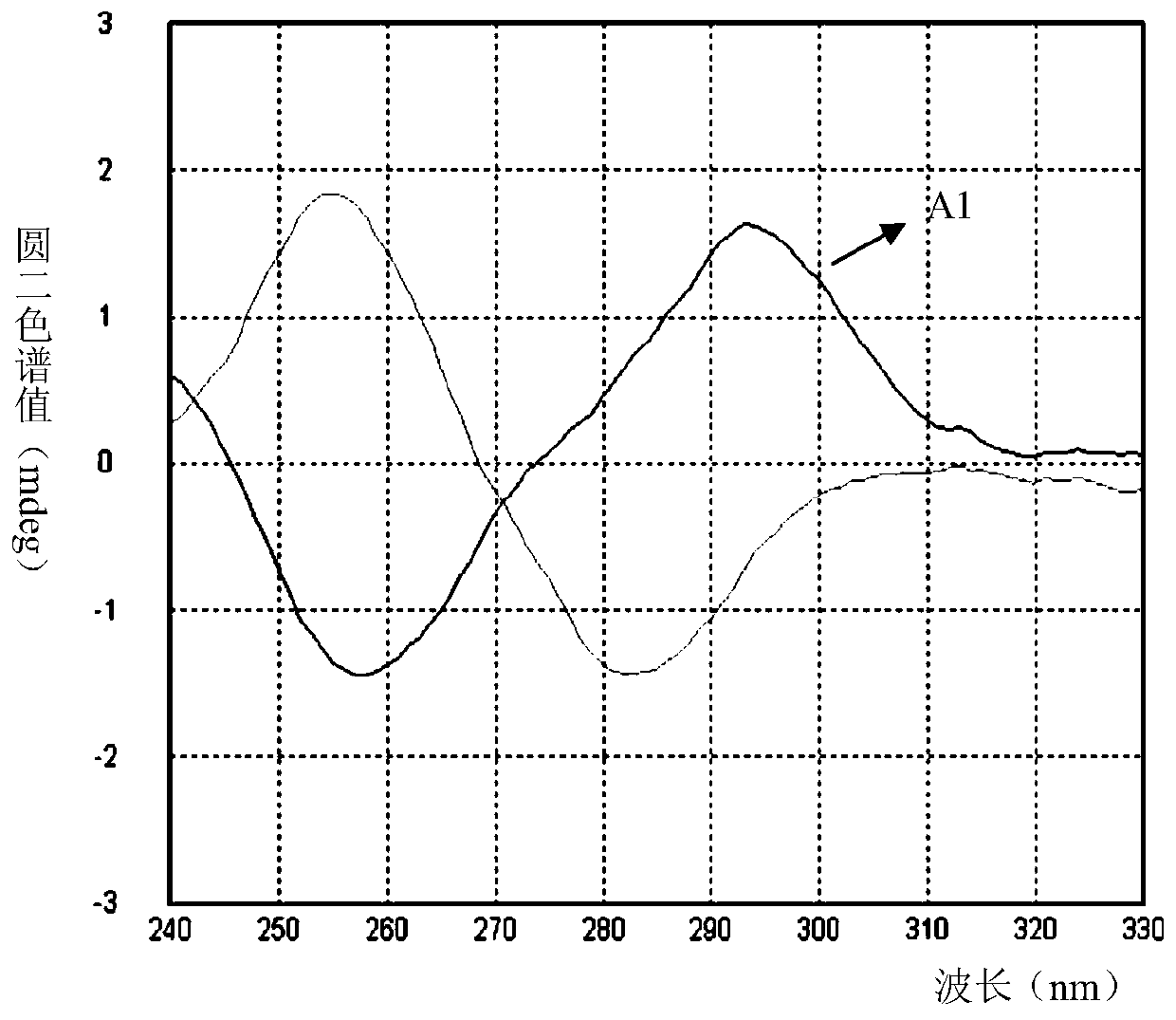
[0052] Example 2: (E)-2-(4-aminostyryl)-3-methylbenzo[d]thiazole-3-iodide (compound A1)
[0053]
[0054] Dissolve 150mg (0.52mmol) of compound 2 and 75mg (0.62mmol) of 4-aminobenzaldehyde in methanol, add dropwise 21μl (0.26mmol) of pyridine, heat to reflux for 12h, cool to room temperature, filter with suction, wash with methanol and After washing with a small amount of ether, 178 mg of a purple solid was obtained. The yield is 87%. 1 H NMR (400MHz, DMSO) δ8.29(d, J=8.0Hz, 1H), 8.08(d, J=8.4Hz, 1H), 7.99(d, J=15.3Hz, 1H), 7.81-7.75(m ,3H),7.67(t,J=7.6Hz,1H),7.55(d,J=15.3Hz,1H),6.68(d,J=8.7Hz,4H),4.21(s,3H); 13 C NMR(101MHz,DMSO)δ171.85,155.20, 150.95,142.39,133.92,129.29,127.84,127.17,124.25,122.03,116.35,114.32, 106.04,36.02. ESI-MS(m / z):267. + .
Embodiment 3
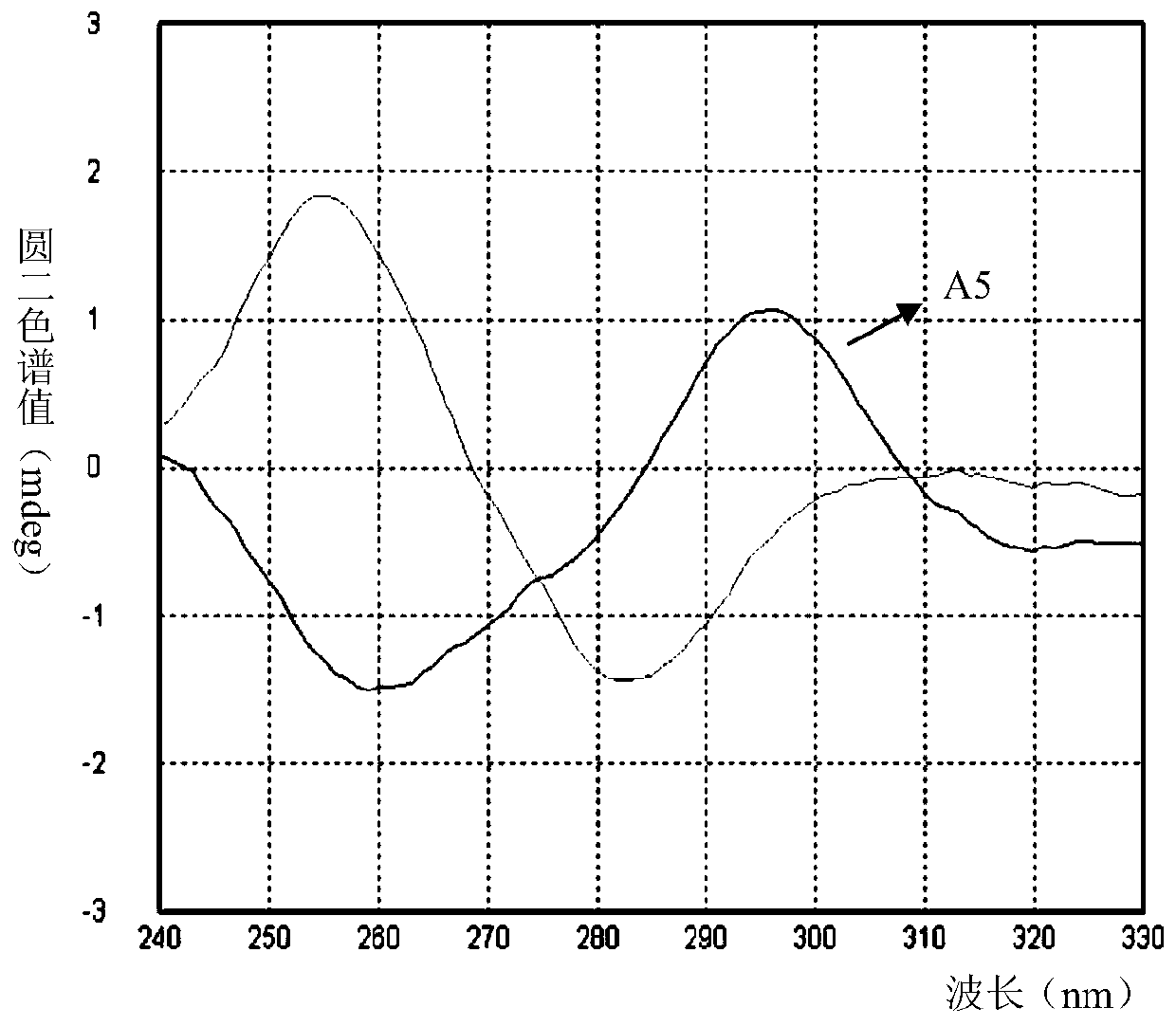
[0055] Example 3: (E)-2-(4-(dimethylamino)styryl)-3-methylbenzo[d]thiazole-3-iodide (Compound A2)
[0056]
[0057] Using compound 2 as raw material, the preparation method is the same as in Example 2, the only difference being that 75mg (0.62mmol) of 4-aminobenzaldehyde is replaced by 92mg (0.62mmol) of 4-(dimethylamino)benzaldehyde to obtain purple Solid A2 in 83% yield. 1 H NMR (400MHz, DMSO) δ8.30(d, J=8.0Hz, 1H), 8.08(dd, J=14.5, 12.0Hz, 2H), 7.91(d, J=8.9Hz, 2H), 7.79(t ,J=7.8Hz,1H),7.68(t,J=7.6Hz,1H),7.62(d,J=15.3Hz,1H),6.83(d,J=8.9Hz,2H),4.23(s,3H ),3.11(s,6H); 13 C NMR (101MHz, DMSO) δ171.80, 153.96, 150.57, 142.41, 133.29, 129.32, 127.89, 127.27, 124.27, 121.93, 116.40, 112.42, 106.73, 100.00, 36.07. ESI-MS (m / z) + .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com