Preparation method of monobenzo 21-crown-7 and derivative thereof
A kind of derivative and monobenzene technology, applied in the field of preparation of monobenzo-21-crown-7 and its derivatives, can solve the problem of low yield, limited large-scale application, monobenzo-21-crown-7 and its derivatives. Derivative synthesis and processing are cumbersome and other problems, to achieve the effect of good water solubility and easy post-processing
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
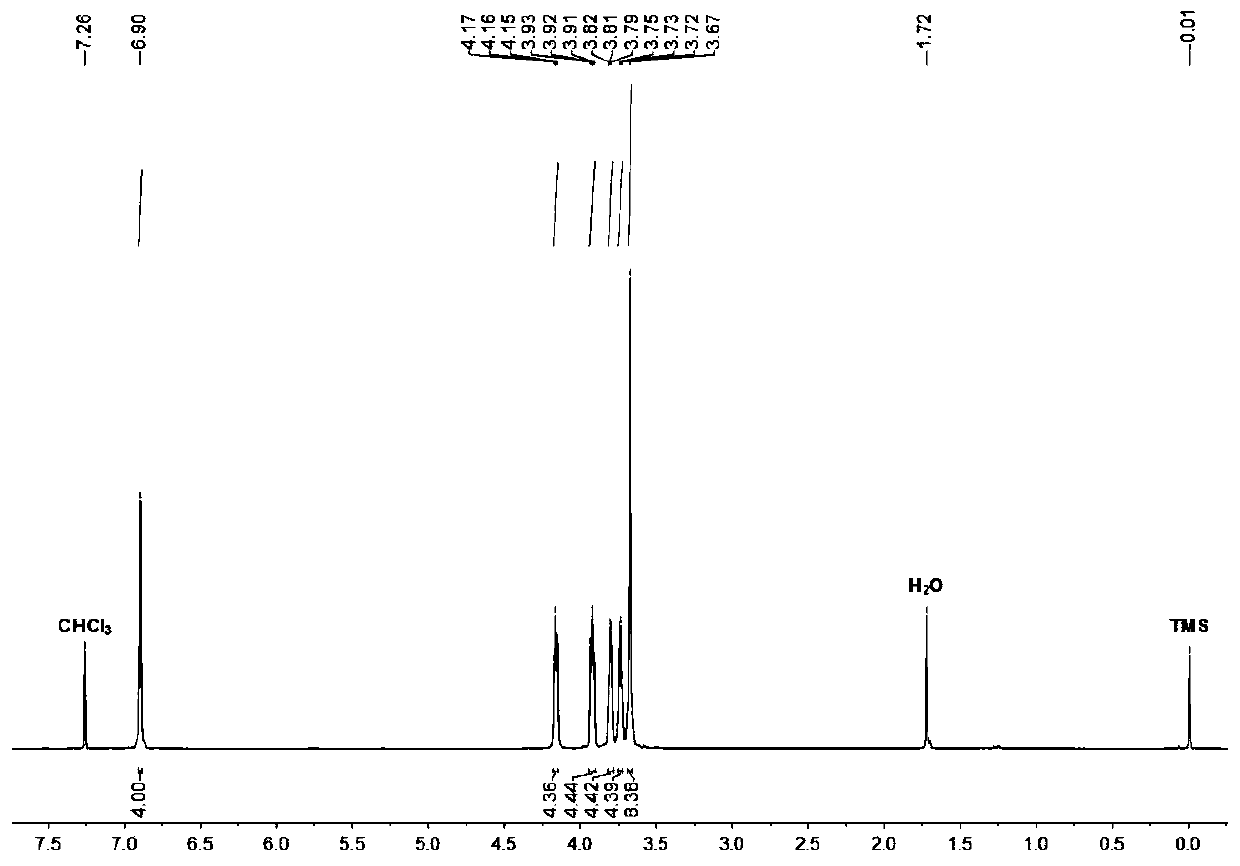
[0034] Synthesis of monobenzo 21-crown-7: 1.1 grams of catechol, 5.91 grams of hexaethylene glycol bis-p-toluenesulfonate, and 5.22 grams of potassium carbonate were reacted at 60°C for 48 hours in 350 milliliters of acetonitrile, and the reaction ended Afterwards, filter while it is hot, remove acetonitrile from the filtrate with a rotary evaporator, dissolve the residue with 250 ml of dichloromethane, wash with water twice, and then remove the dichloromethane with a rotary evaporator to obtain monobenzo 21-crown-7 as 3.23 grams, yield 91%.
[0035] The hydrogen spectrum data are as follows:
[0036] 1 H NMR (400MHz, deuterated chloroform, room temperature): δ6.90(s,4H),4.16(m,4H),3.92(m,4H),3.81(m,4H),3.73(m,4H),3.67 (m,8H).
[0037] Purification: Take 1 gram of the monobenzo21-crown-7 synthesized above, dissolve it in 100 ml of water, stir at room temperature for 12 hours, and then filter it. The obtained filtrate is distilled off under reduced pressure to obtain monoben...
Embodiment 2
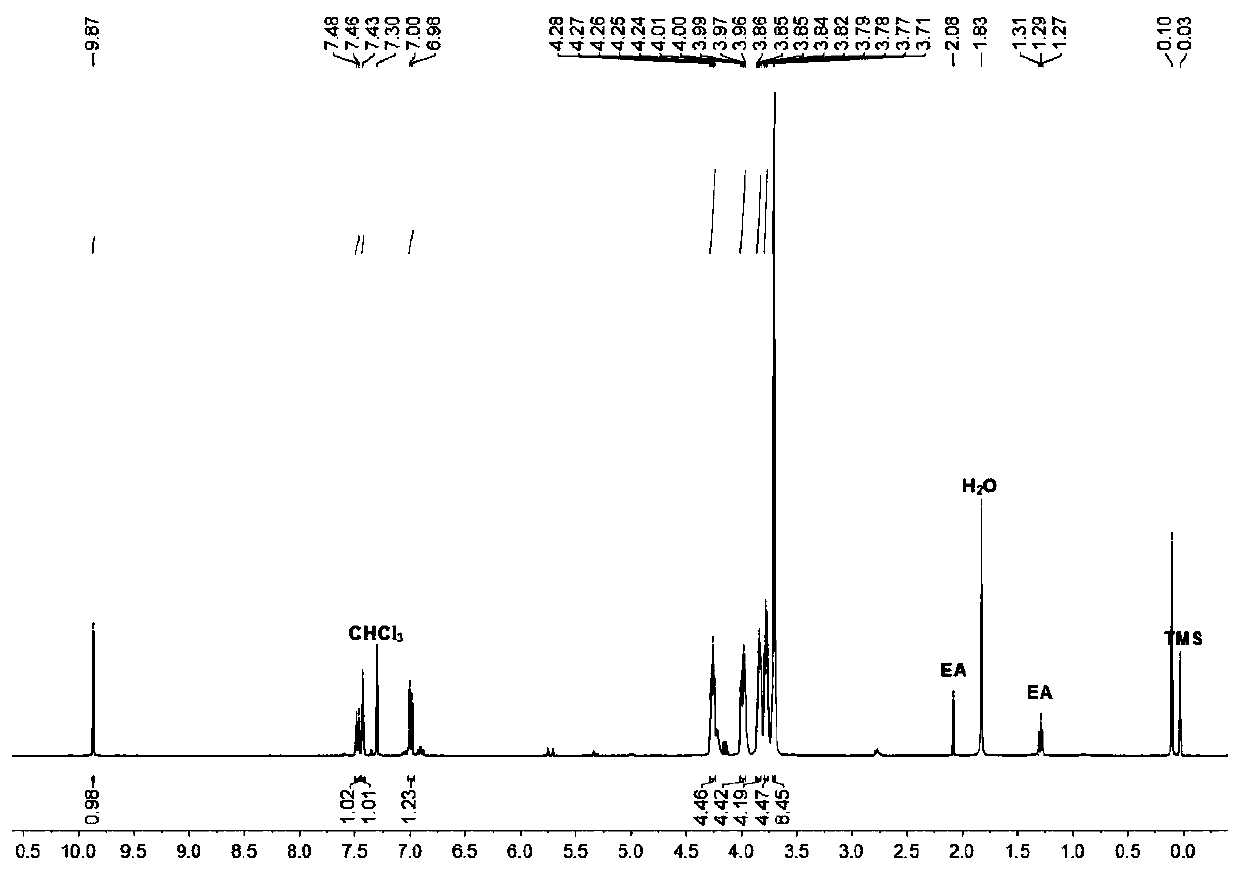
[0041] Synthesis of monobenzo 21-crown-7 aldehyde: 1.38 grams of 3,4-dihydroxybenzaldehyde, 5.91 grams of hexaethylene glycol bis-p-toluenesulfonate, and 5.22 grams of potassium carbonate were reacted at 80°C in 350 milliliters of acetonitrile After 48 hours, after the reaction was over, filter while it was hot, remove the acetonitrile from the filtrate with a rotary evaporator, dissolve the residue with 250 ml of dichloromethane, wash with water twice, and then remove the dichloromethane with a rotary evaporator to obtain monobenzo 21- Crown-7 aldehyde is 3.46 grams, and the yield is 90%.
[0042] The hydrogen spectrum data are as follows:
[0043] 1 H NMR (400MHz, deuterated chloroform, room temperature): δ9.87(s, 1H), 7.47(d, J=4Hz, 1H), 7.43(s, 1H), 6.99(d, J=4Hz, 1H), 4.26 (m, 4H), 3.97 (m, 4H), 3.84 (m, 4H), 3.78 (m, 4H), 3.71 (m, 8H).
[0044] Purification: Take 1 gram of the monobenzo 21-crown-7 aldehyde synthesized above, dissolve it in 100 ml of water, stir at roo...
Embodiment 3
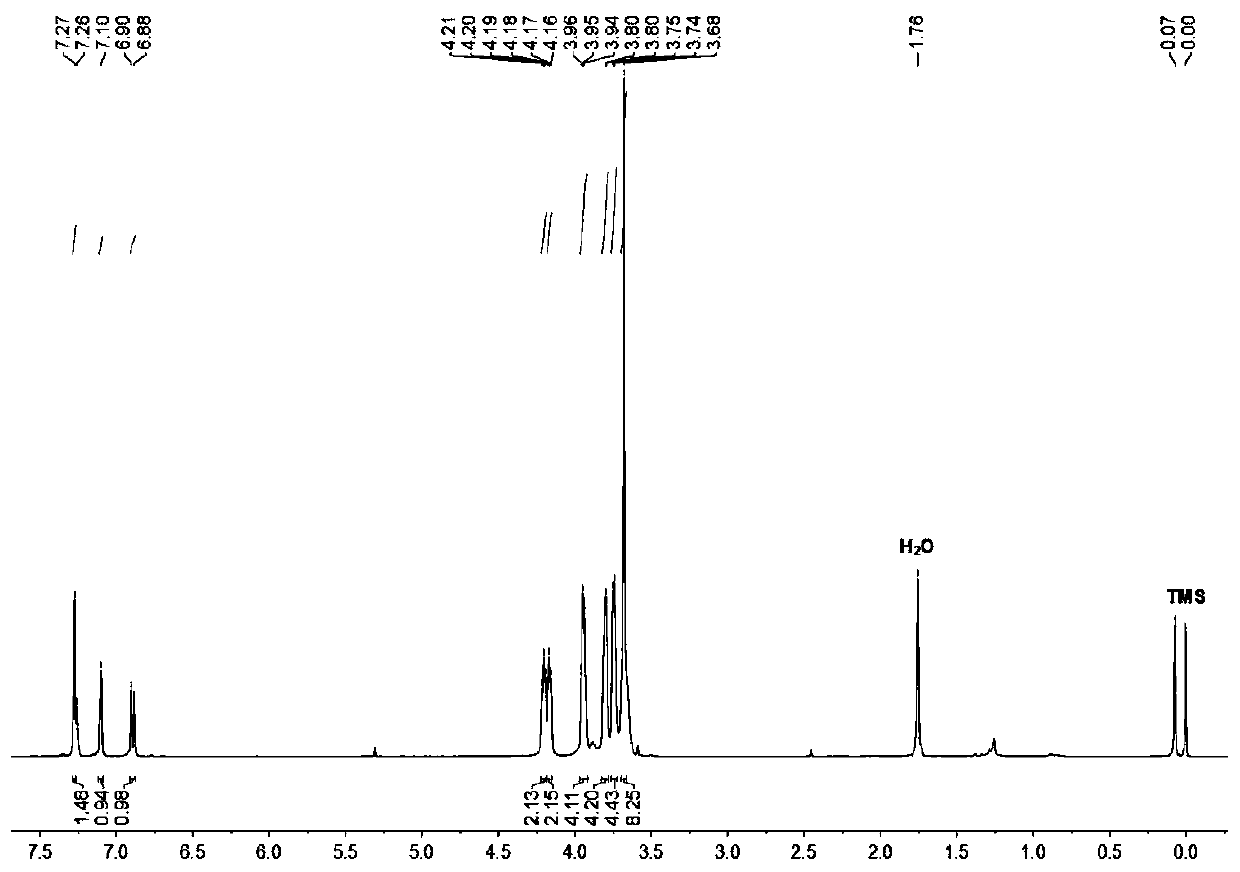
[0048] Synthesis of monobenzo 21-crown-7 nitrile: 1.35 grams of 3,4-dihydroxybenzonitrile, 5.91 grams of hexaethylene glycol bis-p-toluenesulfonate, 5.22 grams of potassium carbonate in 350 ml of acetonitrile at 70 ° C React for 48 hours, after the reaction is over, filter while it is hot, use a rotary evaporator to remove acetonitrile from the filtrate, dissolve the residue with 250 ml of dichloromethane, wash with water twice, and then remove the dichloromethane by a rotary evaporator to obtain monobenzo 21 -Crown-7 nitrile is 3.58 grams, and the yield is 94%.
[0049] The hydrogen spectrum data are as follows:
[0050] 1 H NMR (400MHz, deuterated chloroform , room temperature): δ7.27(s,1H)7.10(s,1H)6.99(d,J=4Hz,1H)4.20(m,2H),4.17(m,2H),3.95(m,4H),3.80( m,4H), 3.75(m,4H), 3.68(m,8H).
[0051] Purification: Take 1 gram of the monobenzo 21-crown-7 nitrile prepared by the above synthesis, dissolve it in 100 ml of water, stir at room temperature for 24 hours and then filter,...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com