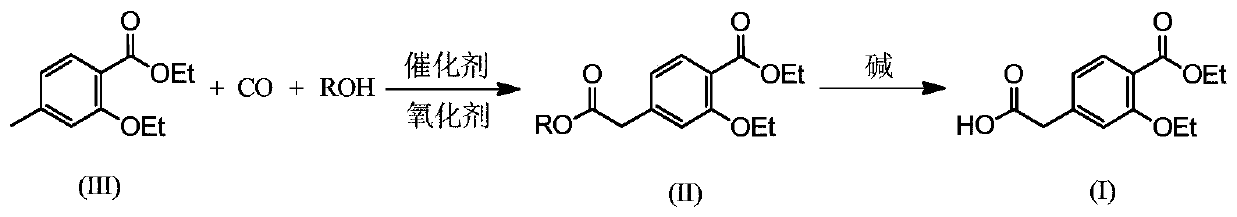
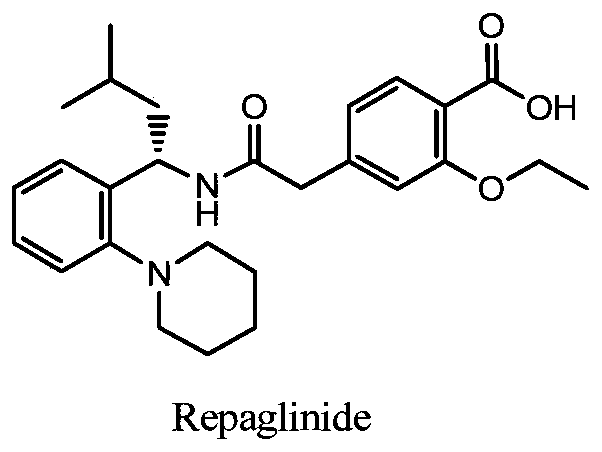
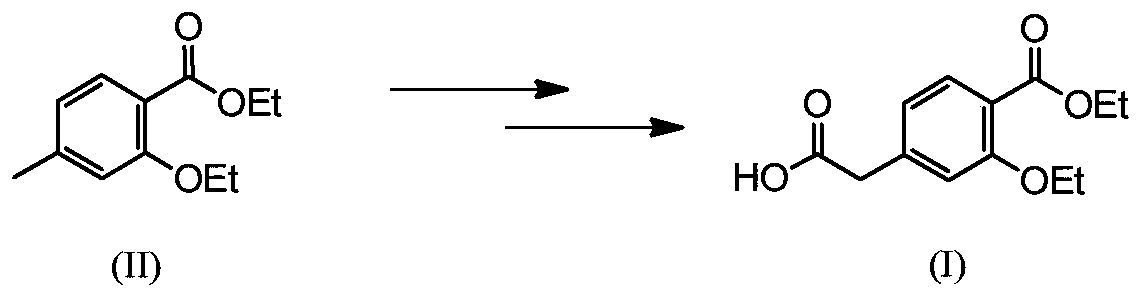
Preparation method of repaglinide key intermediate
A technology for intermediates and carbon monoxide, applied in the field of synthesis of repaglinide intermediates, can solve the problems of harsh reaction conditions, difficult operation, unstable process, etc., and achieves simple operation, easy control, and good reaction reproducibility. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] Add 4-methyl-2-ethoxy ethyl benzoate (2.08g, 10mmol), ethanol (23g, 0.50mol), tetrahydrofuran (100mL), tert-butyl peroxy ether (1.75g, 10mmol) successively in the reaction kettle , PdCl 2 (54.3mg, 0.3mmol) and 1,2-bis(di-2-pyridylphosphine)ethane (123.3mg, 0.3mmol), charged with 2.0MPa of CO, heated to reflux, and stirred for 20 hours. Cool to room temperature, add 2mol / L sodium hydroxide aqueous solution (30mL) to the reaction solution after pressure relief, stir and react for 6 hours, add cyclohexane (50mL×3) to wash, the reaction solution is neutralized to pH 7 with hydrochloric acid, reduce Concentrate under reduced pressure to about 50 mL, acidify to pH 3 with hydrochloric acid, precipitate a yellow solid, filter, and recrystallize the filter residue with toluene-petroleum ether to obtain white solid intermediate (I) (1.94 g, yield: 76.9%).
Embodiment 2
[0042] In the reaction kettle, add 4-methyl-2-ethoxy ethyl benzoate (2.08g, 10mmol), ethanol (80.0g, 1.74mol), potassium persulfate (7.53g, 12mmol), triruthenium dodecacarbonyl (193.8mg, 0.3mmol), 2-bis(diphenylphosphine)methylenepyridine (82.2mg, 0.3mmol), charged with 2.0MPa of CO, heated to reflux, and stirred for 10 hours. After cooling to room temperature, 2 mol / L potassium hydroxide aqueous solution (10 mL) was added to the reaction solution after the pressure was released, and the reaction was stirred for 6 hours. Wash with cyclohexane (50mL×3), neutralize with hydrochloric acid to pH 7, concentrate under reduced pressure to about 50mL, acidify with hydrochloric acid to pH 3, precipitate a yellow solid, filter, and recrystallize the filter residue with toluene-petroleum ether to obtain a white solid Intermediate (I) (2.06 g, yield: 81.7%).
Embodiment 3
[0044] Add 4-methyl-2-ethoxy ethyl benzoate (2.08g, 10mmol), isopropanol (100mL, 1.33mol), 30% hydrogen peroxide (13.6mL, 12mmol), copper fluoride (24.9mL) in the reaction kettle mg, 0.3mmol), bis-(diphenylphosphine)ethylamine (132.9mg, 0.3mmol), filled with 2.0MPa of CO, heated to reflux, and stirred for 10 hours. Cool to room temperature, add 2mol / L potassium hydroxide aqueous solution (30mL) to the reaction liquid after pressure relief, stir and react at 40°C for 6 hours, add cyclohexane (50mL×3) to wash, and neutralize the reaction liquid with hydrochloric acid until the pH is 7 , concentrated under reduced pressure to about 50 mL, acidified with hydrochloric acid to pH 3, precipitated a yellow solid, filtered, and recrystallized the filter residue with toluene-petroleum ether to obtain white solid intermediate (I) (1.87 g, yield: 74.1%).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com