O-allylation method of alpha,beta-diaryl substituted ethanol
A technology of allylation and diaryl, which is applied in the field of organic synthesis technology, can solve the problems of increased process cost, high reaction temperature, and difficult control, and achieve the effects of less raw material residue, low reaction cost and high economy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] O-allylation of substrate S1:
[0041]
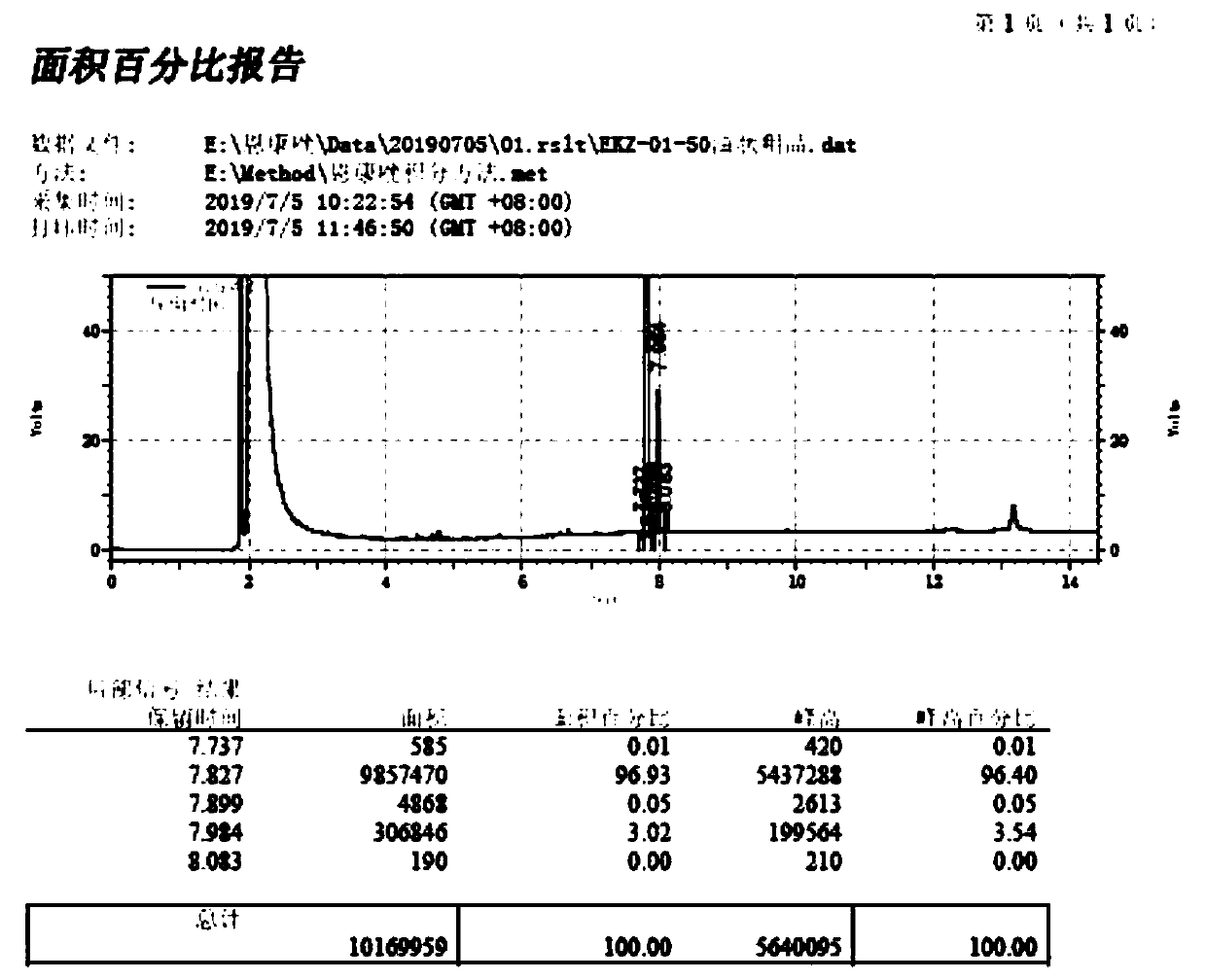
[0042] Dissolve substrate S1 (25.71g, 100mmol) in 100mL dimethyl sulfoxide at room temperature, dissolve 8.42g (150mmol) potassium hydroxide in 22mL water, wait until the temperature drops to room temperature, and then slowly add it dropwise to the above S1 In the dimethyl sulfoxide solution, the temperature of the mixed solution was controlled below 40°C during the dropwise addition, and the reaction was stirred at 35°C for 1 hour after the dropwise addition was completed. Then slowly drop into it a chloropropene solution (8.42g chloropropene is dissolved in 25mL dimethyl sulfoxide), control the rate of addition to maintain the temperature of the reaction solution at 35-40°C, and after the dropwise addition is completed, the reaction temperature will be 35-40°C. Continue to stir the reaction under low temperature. When the liquid phase detection shows that the remaining raw materials are less than 2%, the system is cooled to ...
Embodiment 2
[0045] O-allylation of substrate S2:
[0046]
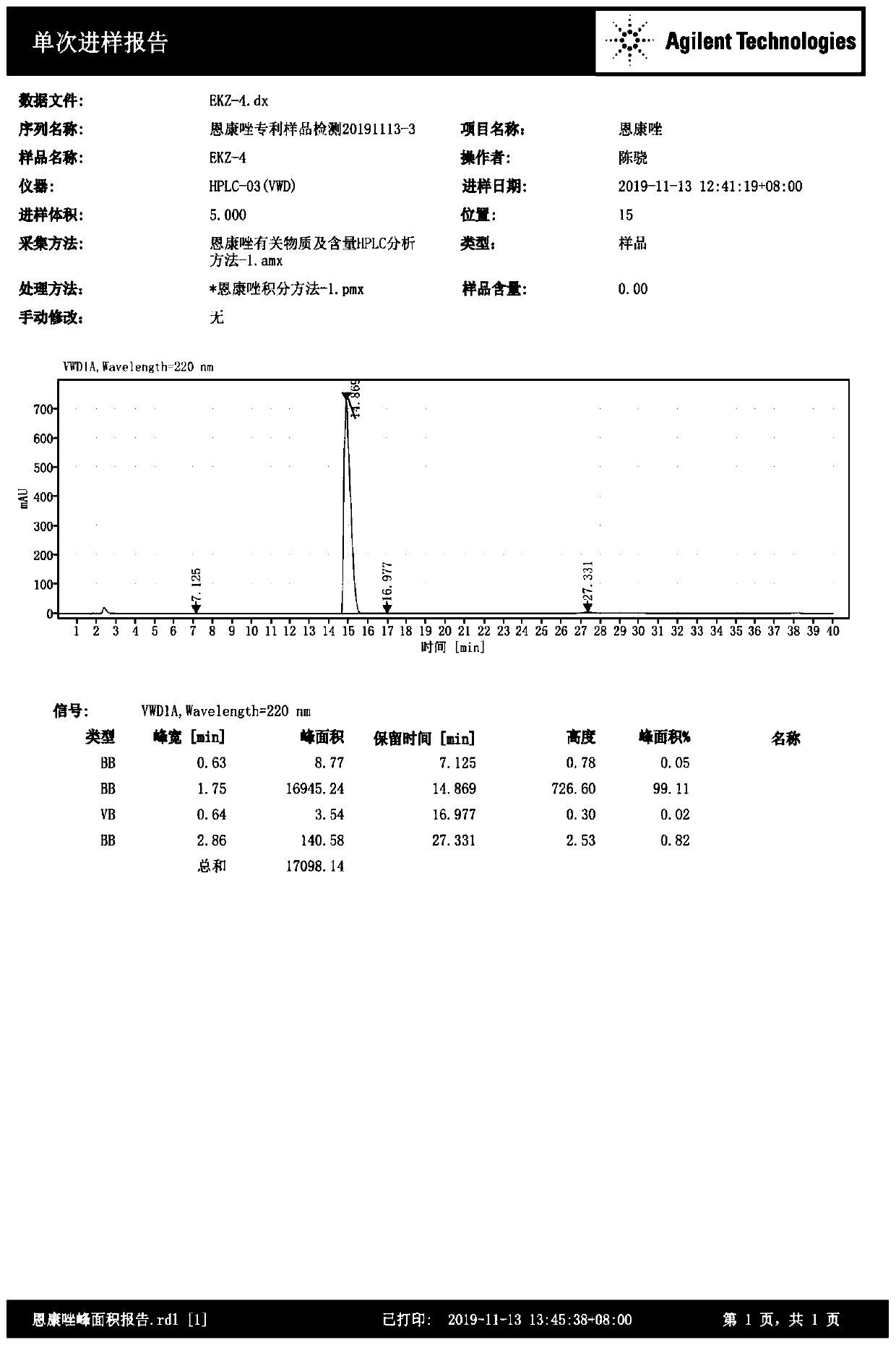
[0047] Substrate S2 (3.76g, 20mmol) was dissolved in 20mL dimethyl sulfoxide at room temperature, 1.60g (40mmol) sodium hydroxide was dissolved in 5mL water and cooled to room temperature, then slowly added dropwise to the above S2 dimethyl In the base sulfoxide solution, the temperature of the mixed solution was controlled below 40°C during the dropwise addition, and the reaction was stirred at 35°C for 1 hour after the dropwise addition was completed. Then slowly drop into it a chloropropene solution (1.70g chloropropene dissolved in 5mL dimethyl sulfoxide), control the rate of addition to maintain the temperature of the reaction solution at 35-40°C, Continue to stir the reaction, after the TLC detection of complete conversion of the raw materials, cool the system to 0°C, add water to quench the reaction, then extract with ethyl acetate, combine the organic phases, wash with salt water, wash with water, dry over anhydrous so...
Embodiment 3
[0049]O-allylation of substrate S5:
[0050]
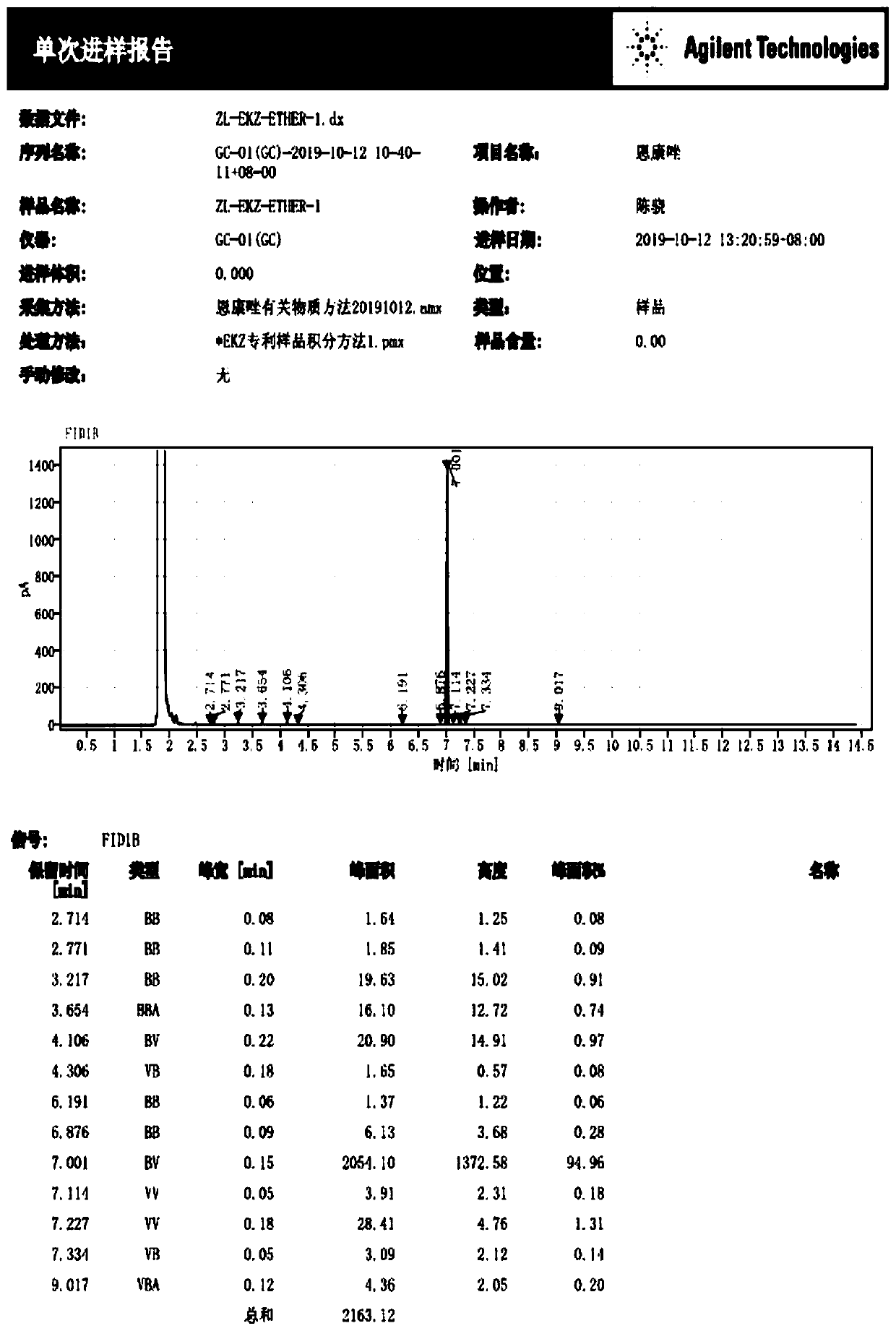
[0051] Substrate S5 (2.23g, 10mmol) was dissolved in 20mL dimethyl sulfoxide at room temperature, 0.84g (15mmol) potassium hydroxide was dissolved in 5mL water and cooled to room temperature, then slowly added dropwise to the above S5 dimethyl In the base sulfoxide solution, the temperature of the mixed solution was controlled below 40°C, and the reaction was stirred at 35°C for 1 hour after the dropwise addition was completed. Then slowly drop allyl chloride solution (1.00g allyl chloride dissolved in 5mL dimethyl sulfoxide) to it, control the rate of addition to maintain the temperature of the reaction solution at 35-40°C, Continue to stir the reaction, after the TLC detection of complete conversion of the raw materials, cool the system to 0°C, add water to quench the reaction, then extract with ethyl acetate, combine the organic phases, wash with salt water, wash with water, dry over anhydrous sodium sulfate, concentrate, an...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com