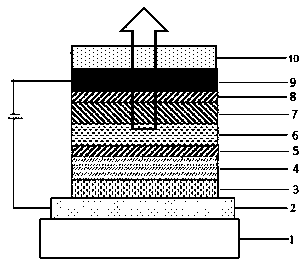
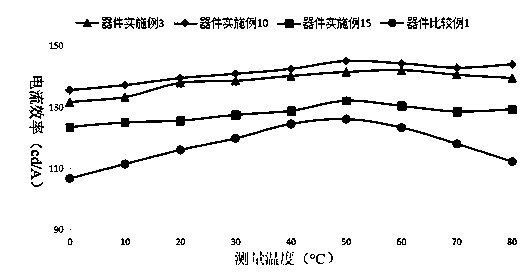
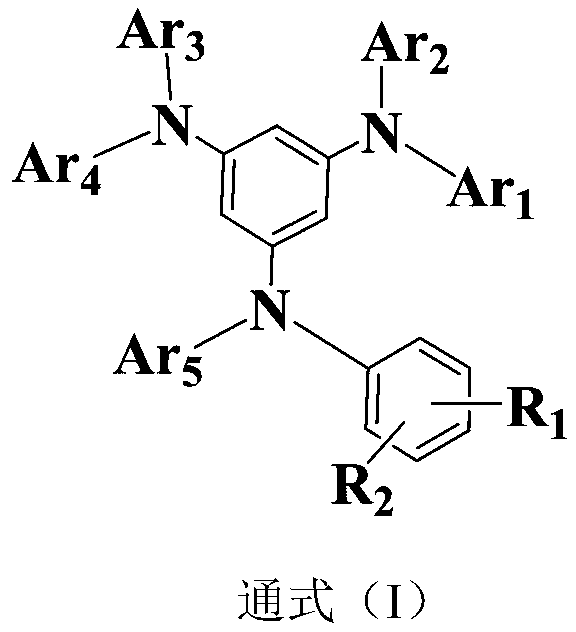
Triarylamine organic compounds and application thereof
A technology of organic compounds and triarylamines, which is applied in the field of triarylamine organic compounds, can solve different problems and achieve the effects of improving current efficiency and life, improving hole injection and transport performance, and improving recombination efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0043] Embodiment 1: the synthesis of intermediate A-1
[0044]
[0045] In a 250ml three-neck flask, under an atmosphere of nitrogen gas, add 0.01mol of raw material I, 0.012mol of raw material II, 0.03mol of potassium tert-butoxide, 1×10 -4 molPd 2 (dba) 3 , 1×10 -4mol triphenylphosphine, 150ml toluene, reflux at 130°C for 12 hours, take a sample and point the plate, and the reaction is complete; natural cooling, filtration, rotary evaporation of the filtrate, passing through a silica gel column, intermediate A-1 is obtained; elemental analysis structure (molecular formula C 21 h 19 N): theoretical value C, 88.38; H, 6.71; N, 4.91; tested value: C, 88.39; H, 6.70; N, 4.91. ESI-MS(m / z)(M + ): The theoretical value is 285.39, and the measured value is 285.44.
[0046] Intermediate A was prepared by the synthesis method of Intermediate A-1, and the specific structure is shown in Table 1.
[0047] Table 1
[0048]
[0049]
Embodiment 2
[0050] Embodiment 2: the synthesis of compound 4
[0051]
[0052] In a 250ml three-necked flask, under a nitrogen atmosphere, add 0.01mol of raw material III, 0.012mol of intermediate A-1, 0.03mol of potassium tert-butoxide, 1×10 -4 molPd 2 (dba) 3 , 1×10 -4 mol triphenylphosphine, 150ml toluene, reflux at 130°C for 12 hours, take a sample and point the plate, and the reaction is complete; natural cooling, filtration, rotary evaporation of the filtrate, passing through a silica gel column, intermediate B-1 is obtained; elemental analysis structure (molecular formula C 21 h 39 BrN 2 ): Theoretical C, 79.66; H, 5.43; Br, 11.04; N, 3.87; Tested: C, 79.68; H, 5.44; Br, 11.02; ESI-MS(m / z)(M + ): The theoretical value is 723.76, and the measured value is 723.85.
[0053] In a 250ml three-neck flask, under a nitrogen atmosphere, add 0.01mol of intermediate B-1, 0.012mol of intermediate A-2, 0.03mol of potassium tert-butoxide, 1×10 -4 molPd 2 (dba) 3 , 1×10 -4 mol triph...
Embodiment 16
[0060] Embodiment 16: the synthesis of compound 212
[0061]
[0062] In a 250ml three-neck flask, under nitrogen atmosphere, add 0.01mol raw material III, 0.012mol intermediate A-3, 0.03mol potassium tert-butoxide, 1×10 -4 molPd 2 (dba) 3 , 1×10 -4 mol triphenylphosphine, 150ml toluene, reflux at 120°C for 10 hours, take a sample and point the plate, and the reaction is complete; natural cooling, filtration, rotary evaporation of the filtrate, passing through a silica gel column, intermediate B-15 is obtained; elemental analysis structure (molecular formula C 18 h 13 Br 2 N): Theoretical C, 53.63; H, 3.25; Br, 39.64; N, 3.47; Found: C, 53.64; H, 3.26; Br, 39.65; N, 3.46. ESI-MS(m / z)(M + ): The theoretical value is 403.12, and the measured value is 403.15.
[0063] In a 250ml three-neck flask, under a nitrogen atmosphere, add 0.01mol of intermediate B-15, 0.012mol of intermediate A-24, 0.03mol of potassium tert-butoxide, 1×10 -4 molPd 2 (dba) 3 , 1×10 -4 mol trip...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com