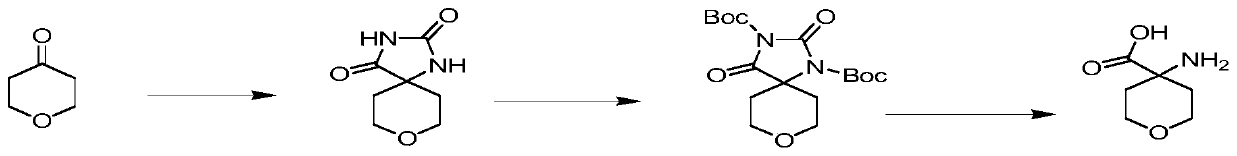
Preparation method of 4-amino-tetrahydro-2-pyran-4-carboxylic acid
A technology of tetrahydropyranone and tetrahydrofuran, applied in directions such as organic chemistry, can solve the problems of complicated post-processing, low yield, and difficulty in industrialized production, and achieve the effects of easy purification, simple operation process, and reduced waste
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0049] Embodiment 1: a kind of preparation method of 4-aminotetrahydro-2-pyran-4-carboxylic acid comprises the following steps:
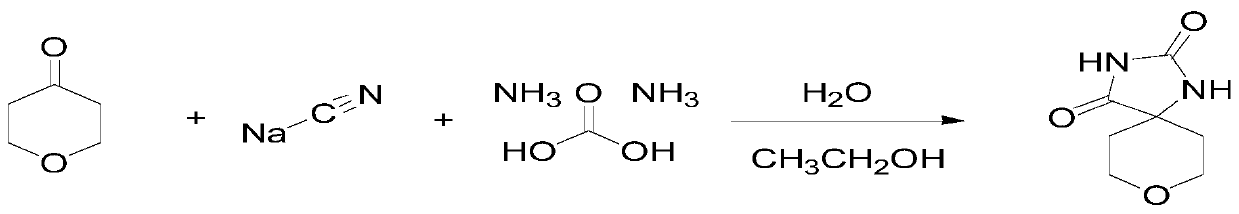
[0050] Step 1. Add 100g of tetrahydropyrone into 500mL of deionized water and 500mL of ethanol, then add 247g of ammonium carbonate, add 89g of sodium cyanide, heat to 60°C for 3 hours, cool to 5°C, and filter Dry under vacuum at 70°C for 4 hours, wash the filter cake twice with ice water at -1.5°C to obtain a white solid, and dry to obtain 165g of Intermediate 1.
[0051] 1H NMR(DMSO-d6):10.35(br s,1 H),8.59(s,1 H),3.76(dt,2H),3.55(td,2H),1.79(ddd,2H),1.43- 1.41 (m, 2 H), according to the inspection data of proton nuclear magnetic resonance spectrum, it can be known that the chemical structural formula of the above-mentioned intermediate one is: Simultaneously, the chemical reaction equation of step 1 is as figure 2 shown.
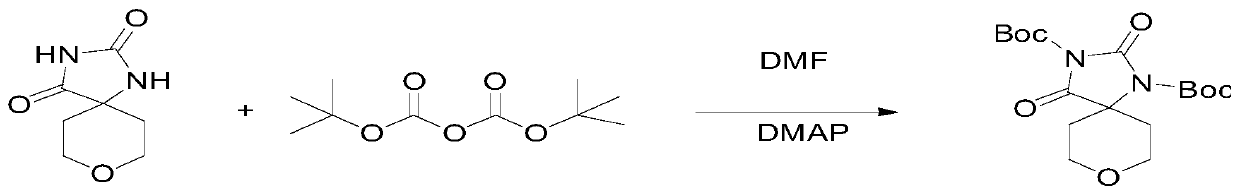
[0052] Step 2: Add 165g of intermediate 1 to 2L of DMF, then add 5g of DMAP, dropwise add 465g of di-tert-butyl dicarbon...
Embodiment 2
[0057] Embodiment 2: a kind of preparation method of 4-aminotetrahydro-2-pyran-4-carboxylic acid comprises the following steps:
[0058] Step 1. Add 100 g of tetrahydropyrone into 800 ml of water and 800 ml of ethanol, then add 200 g of ammonium carbonate and 50 g of sodium cyanide, heat to 65 ° C for 4 hours, and filter with a suction filter funnel to obtain The filter cake was washed twice with ice water at about 1.5°C to obtain a white solid, which was dried under vacuum at 40°C for 4 hours to obtain 160 g of Intermediate 1.
[0059] Step 2: Add 160g of intermediate 1 into 1.6L of DMF, then add 5g of DMAP, dropwise add 450g of di-tert-butyl dicarbonate, heat up to 90°C, react for 18h, pour into water and stir for 2.5h, filter It was dried under vacuum at 40°C for 5 hours to obtain 310 g of Intermediate II.
[0060] Step 3: Add 310g of intermediate 2 into 1.085L of tetrahydrofuran, then add 775ml of 40wt% sodium hydroxide solution, heat to reflux at 50°C for 6h, distill off...
Embodiment 3
[0061] Embodiment 3: a kind of preparation method of 4-aminotetrahydro-2-pyran-4-carboxylic acid comprises the following steps:
[0062] Step 1. Add 100 g of tetrahydropyrone into 600 ml of water and 600 ml of ethanol, then add 300 g of ammonium carbonate and 120 g of sodium cyanide, heat to 70 ° C for 3 hours, and filter with a suction filter funnel to obtain The filter cake was washed three times with ice water at 0°C to obtain a white solid, which was dried under vacuum at 60°C for 5 hours to obtain 170 g of Intermediate 1.
[0063] Step 2: Add 170g of intermediate 1 into 2.55L of DMF, then add 5g of DMAP, dropwise add 475g of di-tert-butyl dicarbonate, heat up to 100°C, react for 20h, pour into water and stir for 2h, filter and put in It was dried under vacuum at 60°C for 6 hours to obtain 300 g of intermediate 2.
[0064] Step 3: Add 300g of intermediate 2 into 1.86L of tetrahydrofuran, then add 750ml of 60wt% sodium hydroxide solution, heat and reflux at 60°C for 6h, di...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com