Method for preparing quality control product for five-class blood cell analyze
A blood cell analyzer and five-classification technology, applied in the field of quality control material preparation for five-class blood cell analyzers, can solve the problems of differences in measured values, difficulty in evaluating and interpreting results, etc., achieving low production costs, filling domestic gaps, and ensuring The effect of consistency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
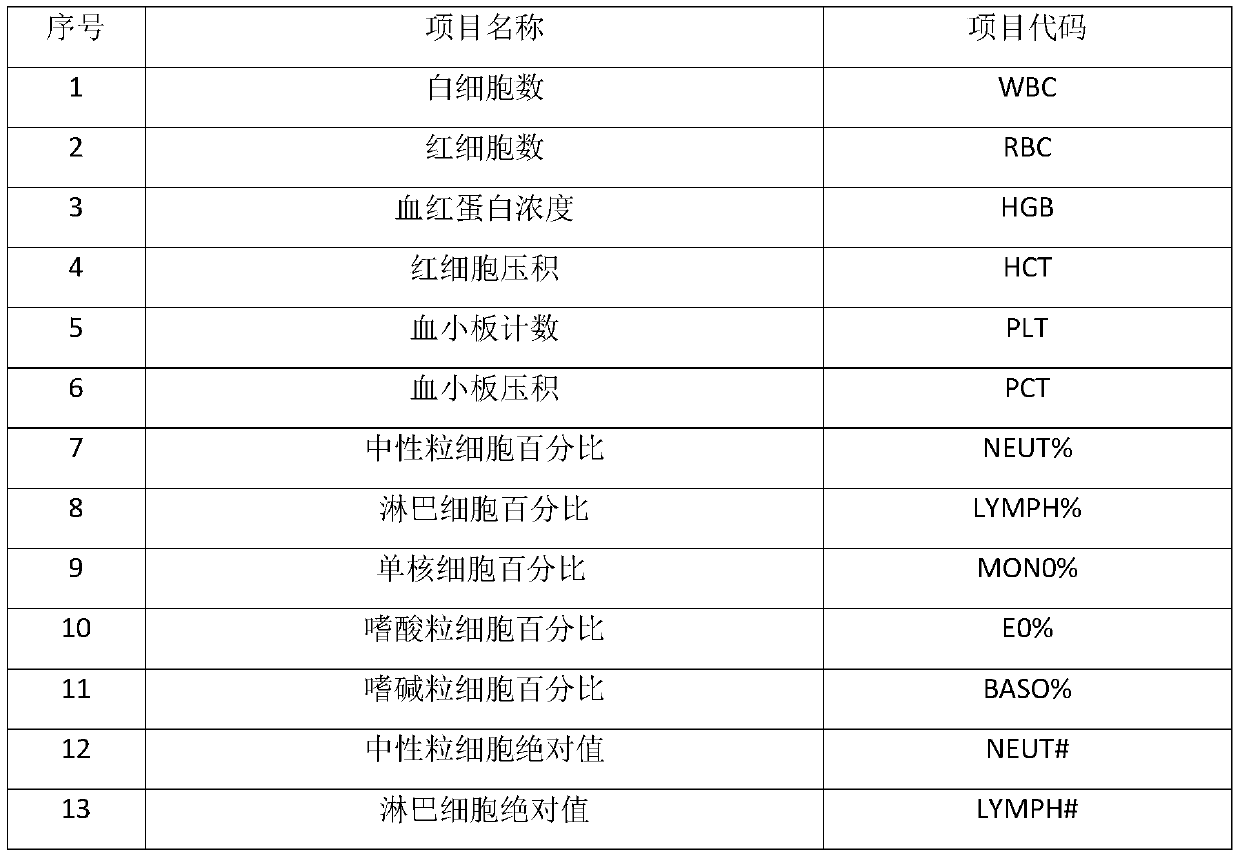
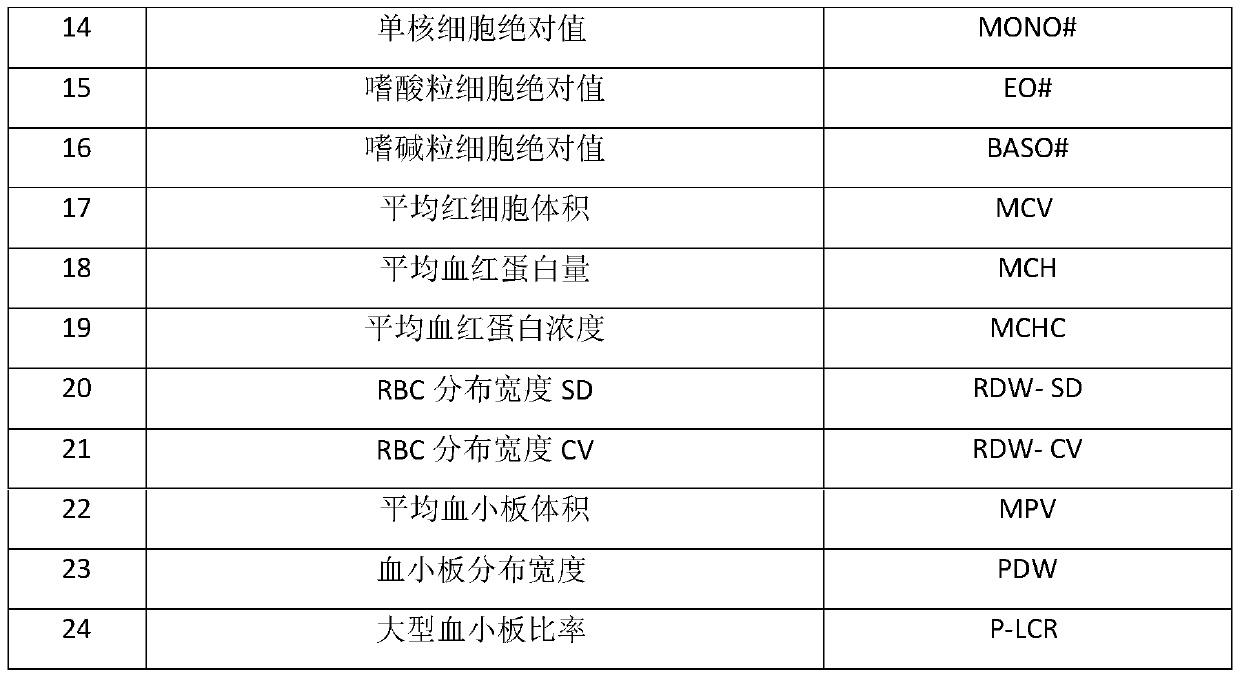
[0027] 1. A method for preparing a quality control product for a five-differentiation hematology analyzer
[0028] The first step, the preparation of fixative:
[0029] Fixative solution A: 0.3% formaldehyde, 0.15% glutaraldehyde, 1% methanol;
[0030] Fixative solution B: formaldehyde 0.7%, glutaraldehyde 0.2%, ethanol 2%;
[0031] Fixative solution C: formaldehyde 1%, glutaraldehyde 0.5%, methanol 0.5%, ethanol 2%.
[0032] The second step, the preparation of blood cell preservation solution:
[0033] Trisodium citrate 14.7g, glucose 27.0g, adenine 0.4g, sodium chloride 8.2g, edetate disodium 0.3g, inosine 2.7g, potassium dihydrogen phosphate 0.122g, disodium hydrogen phosphate 0.716 g, 13.8g of sucrose, 1.8g of polyethylene glycol 4000, 0.7g of chloramphenicol, 1.0g of neomycin sulfate, add purified water to 2000ml.
[0034] The third step is to weigh the "O" type fresh blood that has passed the inspection by a professional organization, perform a complete blood cell co...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com