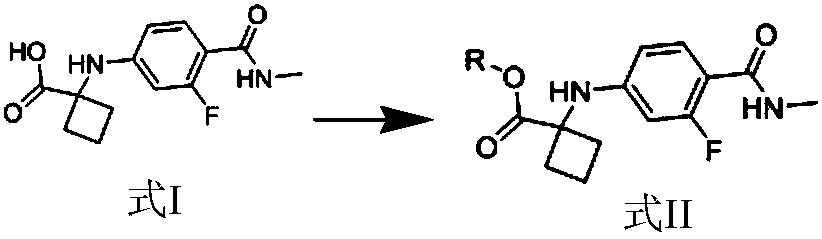
Apalutamide synthetic method and intermediate
A technology of apalutamide and a synthesis method, applied in the field of medicine and chemical industry, can solve the problems of low atom economy and high material cost, and achieve the effects of good application prospect, easy availability of raw materials and mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
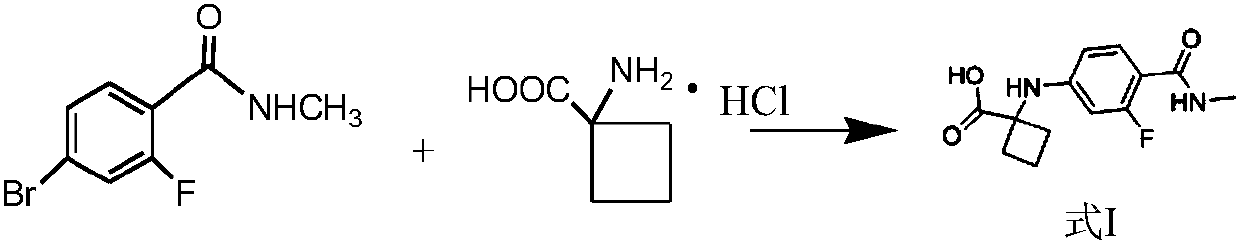
[0059] Example 1 Preparation of 1-((3-fluoro-4-(methylcarbamoyl)phenyl)amino)cyclobutane-1-carboxylic acid (1)
[0060] Drop into 9.28g 2-fluoro-4-bromo-N-methyl benzylamine amide (0.04mol), 9.1g 1-aminocyclobutyl carboxylate hydrochloride (0.06mol), 16.6g salt of wormwood ( 0.12mol), 1.55g CuI (0.008mol), 60mL DMF, and evacuated 3 times with argon protection at room temperature, stirred at room temperature for 30min, replaced with argon, heated to 110°C to react, the solution quickly turned green, and gray appeared after about 2 hours The solid was suspended, and after 9 hours, the plate was spotted, and the raw materials were completely reacted.
[0061] After cooling to room temperature, 100 mL of ethyl acetate / 200 mL of water was added, stirred for 30 min, and separated. The water layer was taken, and the pH of the water layer was adjusted to 4 with 110 mL of 37% citric acid aqueous solution. Solids precipitated out. After stirring in an ice-water bath for 1 h, suction fi...
Embodiment 2
[0066] Example 2 Preparation of 1-((3-fluoro-4-(methylcarbamoyl)phenyl)amino)cyclobutane-1-carboxylic acid (2)
[0067] Drop into 9.28g 2-fluoro-4-bromo-N-methyl benzylamine amide (0.04mol), 9.1g 1-aminocyclobutyl carboxylate hydrochloride (0.06mol), 16.6g salt of wormwood ( 0.12mol), 0.8g CuCl (0.008mol), 60mL DMF, and evacuated 3 times with argon protection at room temperature, stirred at room temperature for 30min, heated to 110°C to react, the solution quickly turned green, and the raw materials reacted completely after 10h.
[0068] After cooling to room temperature, add 100mL ethyl acetate / 200mL water, stir for 30min, separate the layers, take the water layer, adjust the pH of the water layer to 4 with 110mL of 37% citric acid aqueous solution, and solid precipitates, stir in an ice-water bath for 1h, and then filter with suction. After drying, 7.9 g of 1-((3-fluoro-4-(methylcarbamoyl)phenyl)amino)cyclobutane-1-carboxylic acid was obtained, with a yield of 74.1% and a pu...
Embodiment 3
[0069] Example 3 Preparation of 1-((3-fluoro-4-(methylcarbamoyl)phenyl)amino)cyclobutane-1-carboxylic acid (3)
[0070] Drop into 18.6g 2-fluoro-4-bromo-N-methyl benzylamine amide (0.08mol), 18.0g1-aminocyclobutyl carboxylic acid hydrochloride (0.11mol), 21.2g sodium carbonate (0.20 mol), 1.6g CuCl (0.016mol), 100mL DMF, and evacuate the room with argon protection for 3 times, stir at room temperature for 30min, and heat to 110°C to react. After 10h, the raw materials reacted completely.
[0071] After cooling to room temperature, add 250 mL of ethyl acetate / 400 mL of water, stir for 30 min, separate the layers, and take the water layer; The aqueous layer was adjusted to pH ~ 4 with 10% hydrochloric acid, and solids precipitated out. After stirring in an ice-water bath for 1 h, suction filtration and drying gave 15.8 g of 1-((3-fluoro-4-(methylcarbamoyl)phenyl) Amino)cyclobutane-1-carboxylic acid, yield 74%, liquid phase detection purity 98.4%. Spectrogram detection result i...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com