Preparation method of azacitidine crystal form
A technology of azacitidine crystals and azacitidine, which is applied in the field of drug preparation, and can solve problems such as difficulty in drug quality control, inability to produce qualified drugs, and solvent residues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
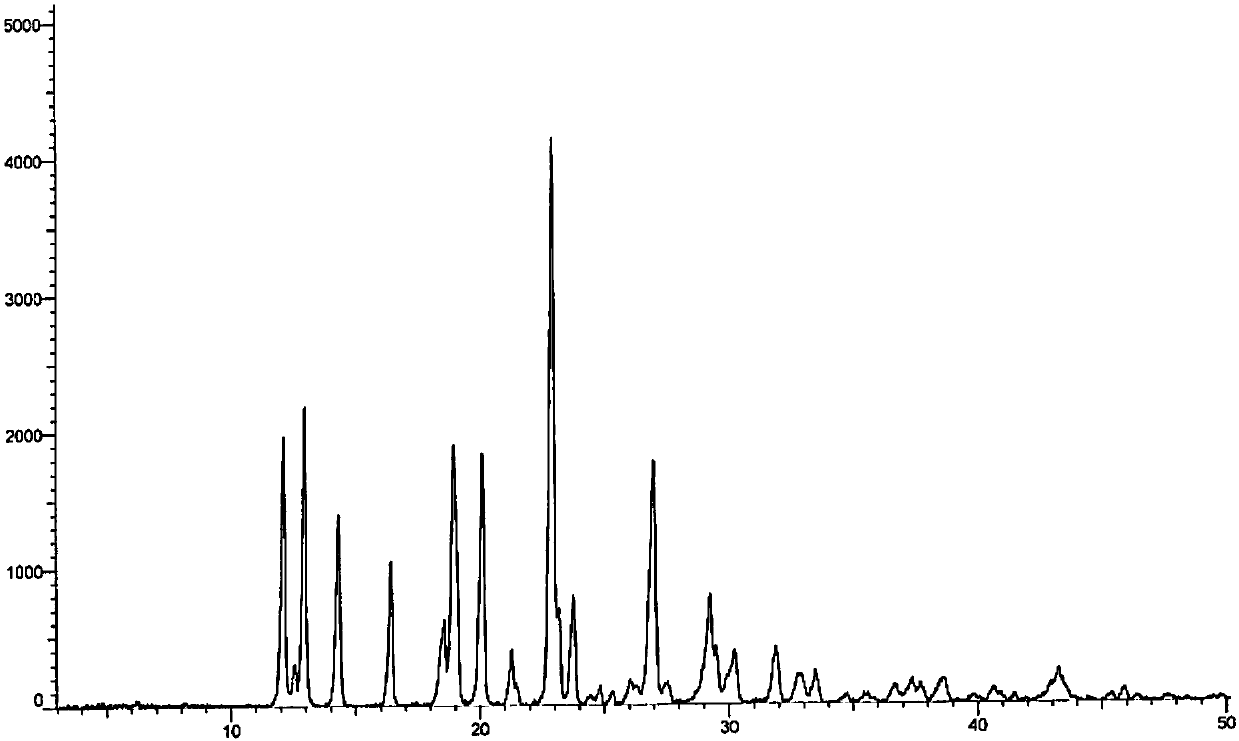
[0049] Mix 1g of azacitidine with 5ml of DMSO, heat to 75°C until the sample is completely dissolved, slowly add 30ml of a mixed solvent consisting of methanol / n-hexanol = 1:1, lower the temperature to room temperature and stir for 1h, filter, and the sample is at 80 After vacuum drying at ℃ for 24 hours, a 0.8g sample of Form I was obtained. The solvent residue in DMSO was measured to be 830ppm, and the purity was greater than 99.9%. The XRD pattern was as follows: figure 1 shown.
Embodiment 2
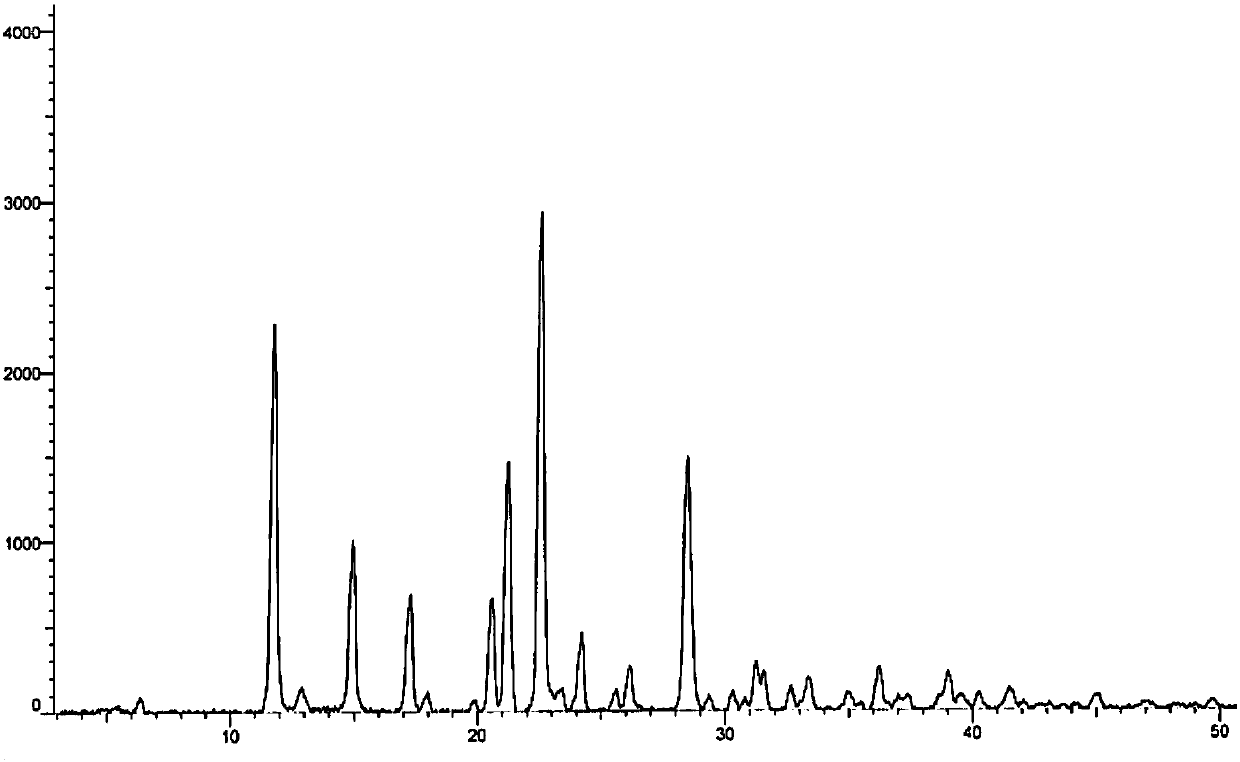
[0051] Mix 10g of azacitidine with 40ml of DMSO, heat to 90°C to dissolve the sample, slowly add 200mL of a mixed solvent consisting of methanol / n-heptanol = 1:1, continue stirring for 1h after the temperature drops to room temperature and filter, sample 70 After drying at ℃ for 24 hours, 9.1 g of a sample of crystal form I was obtained. The residual DMSO solvent was measured to be 1100 ppm, and the purity was greater than 99.9%. The XRD pattern and the attached figure 1 Basically the same.
Embodiment 3
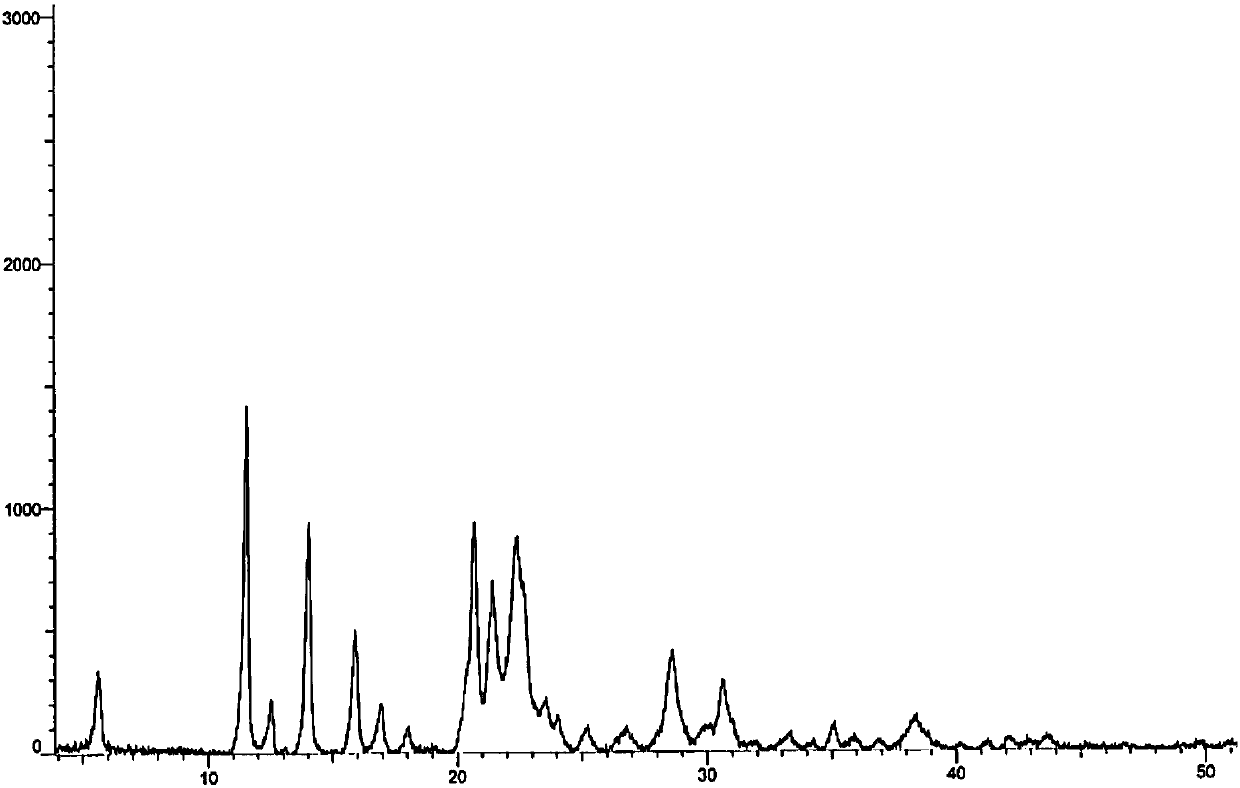
[0053] Mix 1g of azacitidine with 8ml of DMSO, heat to 65°C to dissolve all the samples, slowly add 80ml of a mixed solution composed of ethanol / n-octanol=2:8, lower the temperature to room temperature and continue to stir for 1h to filter, then filter at 75°C Dry under vacuum for 20h to obtain 0.88g of the crystal form I sample, the DMSO solvent residue was measured to be 5722ppm, the XRD pattern and the attached figure 1 Basically the same.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com