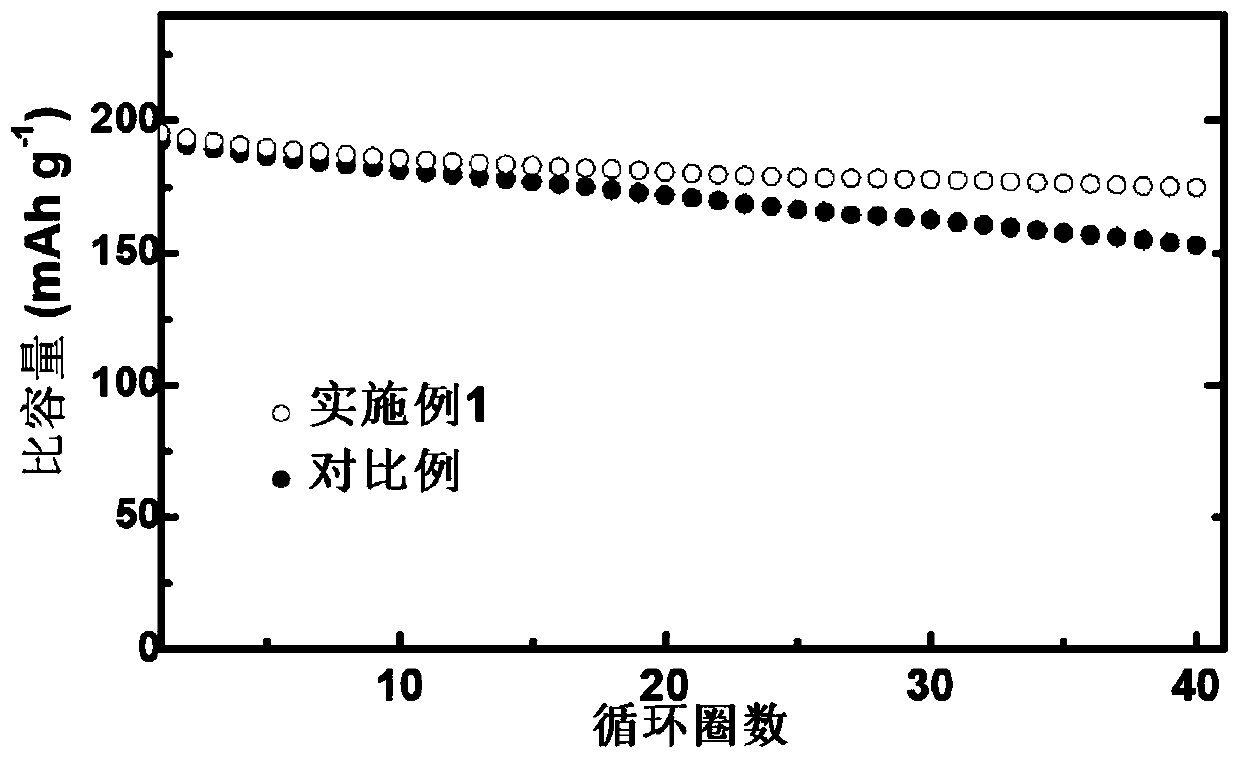
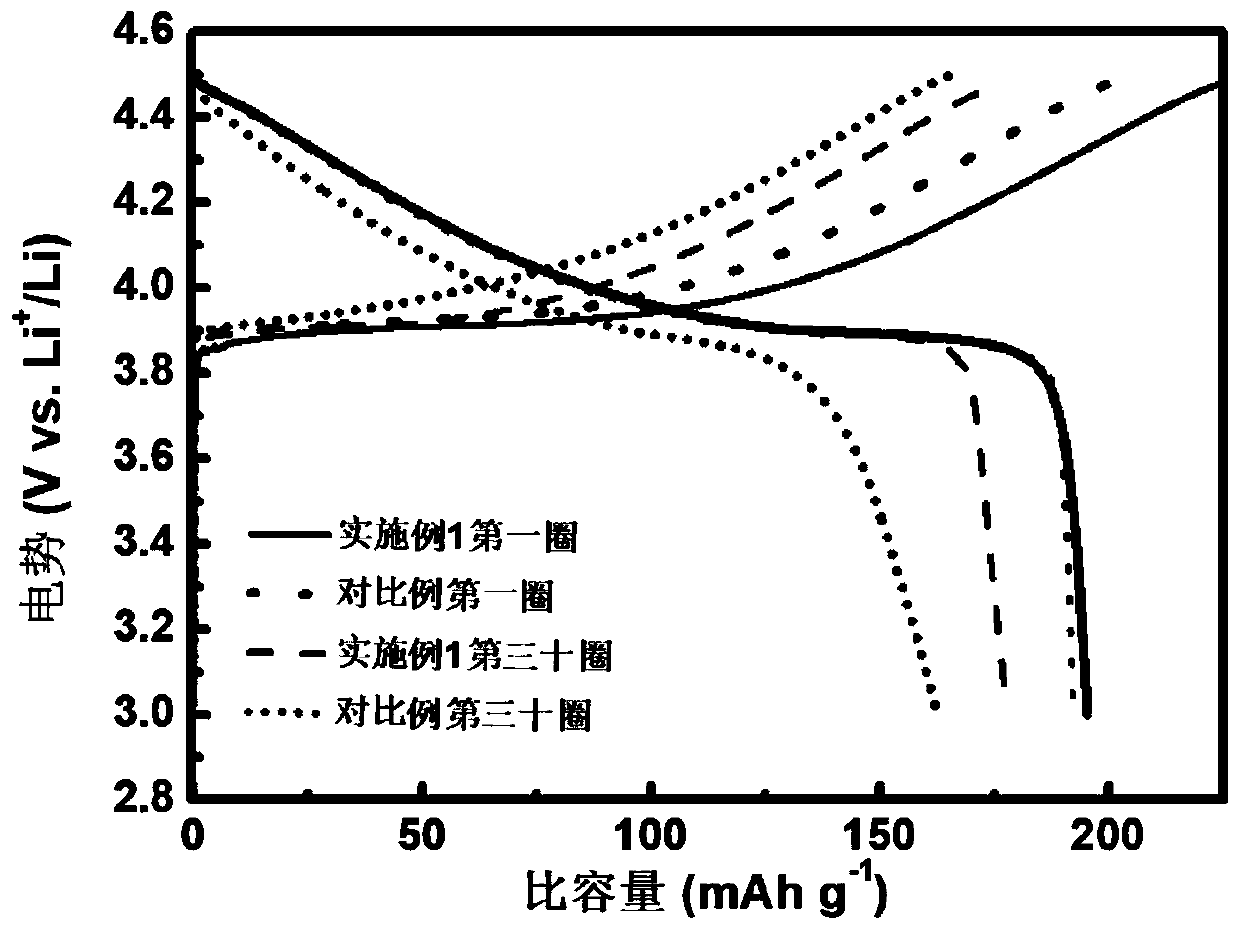
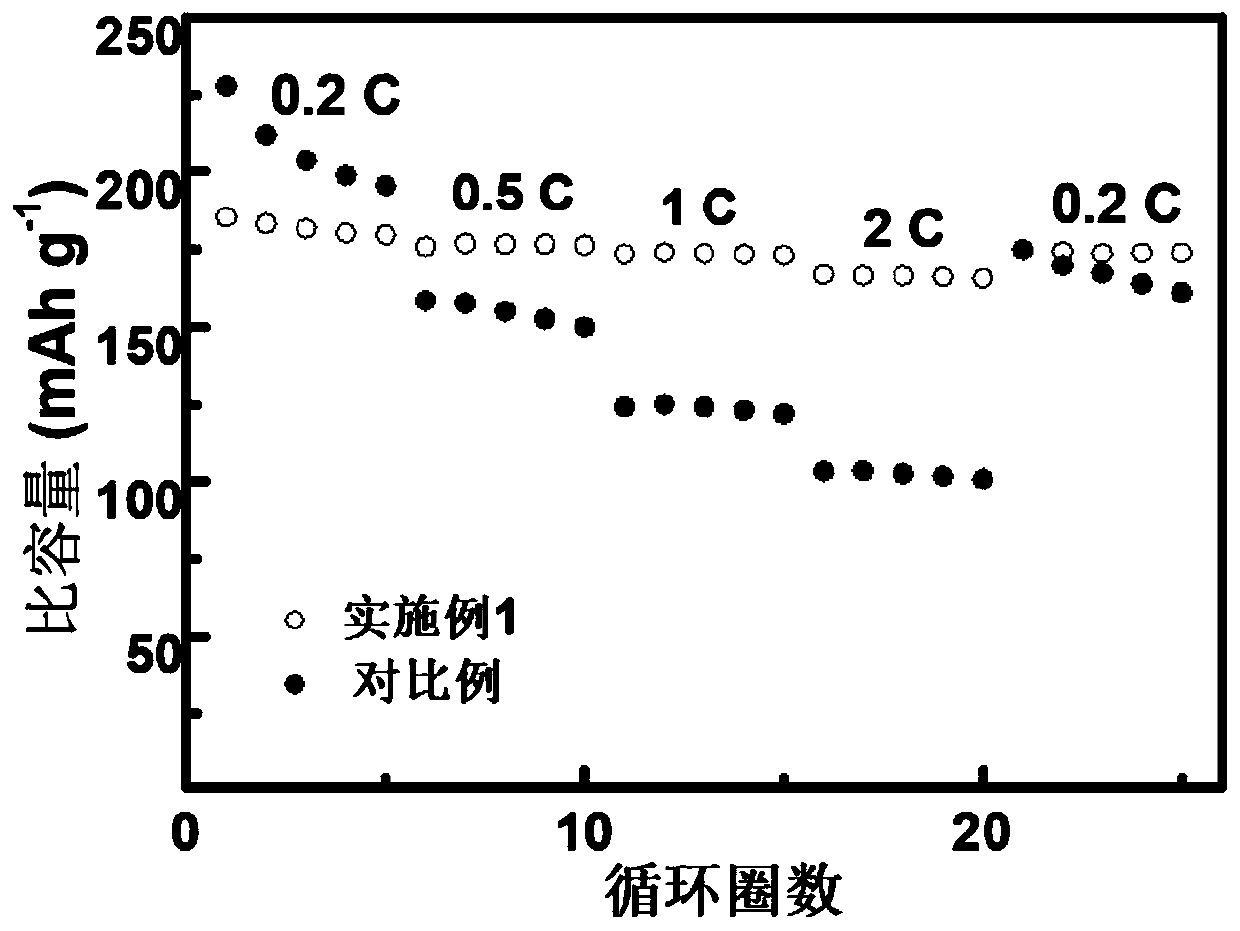
Li1+xAlxTi2-x (PO3)4-coated lithium cobalt oxide material and preparation method and application thereof
A lithium cobalt oxide and coating technology, which is applied in the field of lithium cobalt oxide materials and its preparation, can solve problems such as increased impedance, increased problems, and decreased cycle stability, and achieves reduced polarization, less impact, and reduced interface impedance. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] Li 1+x al x Ti 2-x (PO 4 ) 3 The preparation of coated lithium cobalt oxide material, take Li 1+x al x Ti 2-x (PO 4 ) 3 In x=0.3, first press Li 1.3 al 0.3 Ti 1.7 (PO 4 ) 3 Stoichiometric ratio, weighed 181.08mg C 16 h 36 o 4 Ti, 170.9mg C 6 h 15 o 4 P, 28.56 mg LiNO 3 and 35.22mg Al(NO 3 ) 3 Dissolve in 15ml of ethanol to obtain a precursor solution. The mass ratio of lithium titanium aluminum phosphate to lithium cobaltate is 0.5%:1, weigh lithium cobaltate, disperse lithium cobaltate in it, stir ultrasonically for 1h, and then dry at 80°C for 1h , and then sintered. During the sintering process, the temperature was raised to 650° C. at a rate of 2° C. / min and kept for 6 hours, and finally cooled to room temperature to obtain the coated lithium cobaltate material. The coated lithium cobaltate material is used as the active material, and it is ground with PVDF and super-P at a mass ratio of 8:1:1 for 30 minutes, then dissolved in N-methylpyrrolido...
Embodiment 2
[0028] Li 1+x al x Ti 2-x (PO 4 ) 3 The preparation of coated lithium cobalt oxide material, take Li 1+x al x Ti 2-x (PO 4 ) 3 In x=0.4, first press Li 1.4 al 0.4 Ti 1.6 (PO 4 ) 3 Stoichiometric ratio, weighed 181.08mg C 16 h 36 o 4Ti , 170.9mg C 6 h 15 o 4 P, 28.56mgLiNO 3 and 35.22mg Al(NO 3 ) 3 Dissolve in 15ml of ethanol to obtain a precursor solution. The mass ratio of lithium titanium aluminum phosphate to lithium cobalt oxide is 1%: 1. Weigh the lithium cobalt oxide, disperse the lithium cobalt oxide in it, stir it ultrasonically for 1 hour, and then dry it at 75°C 2h, and then sintering. During the sintering process, the temperature was raised to 550°C at a heating rate of 1°C / min and kept for 6h, and finally cooled to room temperature to obtain the coated lithium cobalt oxide material. The coated lithium cobalt oxide material is used as the active material, and it is ground with PVDF and super-P at a mass ratio of 8:1:1 for 30 minutes, then disso...
Embodiment 3
[0031] Li 1+x Al x Ti 2-x (PO 4 ) 3 The preparation of coated lithium cobalt oxide material, take Li 1+x Al x Ti 2-x (PO 4 ) 3 In x=0.5, first press Li 1.5 Al 0.5 Ti 1.5 (PO 4 ) 3 Stoichiometric ratio, weighed 181.08mg C 16 h 36 o 4 Ti , 170.9mg C 6 h 15 o 4 P, 28.56mgLiNO 3 and 35.22mg Al(NO 3 ) 3 Dissolve in 15ml of ethanol to obtain a precursor solution. The mass ratio of lithium titanium aluminum phosphate to lithium cobaltate is 2%:1, weigh lithium cobaltate, disperse lithium cobaltate in it, stir ultrasonically for 1h, then dry at 85°C for 1h , and then sintered. During the sintering process, the temperature was raised to 750° C. at a rate of 3° C. / min and kept for 6 hours, and finally cooled to room temperature to obtain the coated lithium cobaltate material. The coated lithium cobaltate material is used as the active material, and the coated positive electrode, PVDF and super-P are ground in a mass ratio of 8:1:1 for 30 minutes, then dissolved in ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com