Device and method for producing hydrogen from magnesium hydride
A hydrogen production device and a technology of magnesium hydride, applied in the field of hydrogen production, can solve the problems of elusiveness, fast diffusion, and low hydrogen production efficiency, and achieve extended use times and time, stable and continuous self-circulating hydrogen production, and long-term satisfaction. The effect of smooth requirements
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
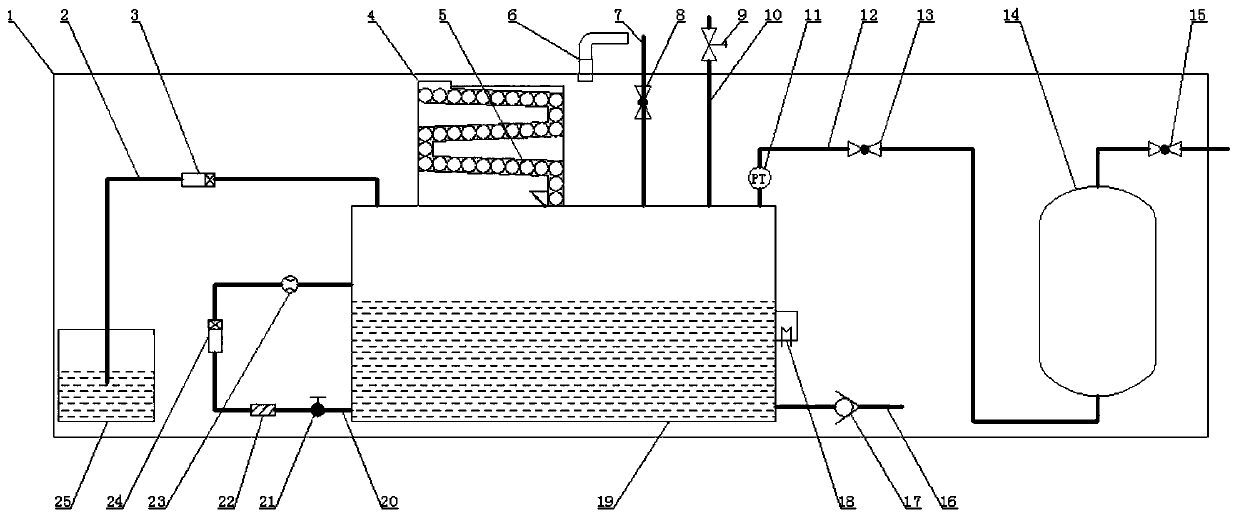
[0042] Such as figure 1 As shown, a hydrogen production device using magnesium hydride as a raw material includes a hydrolysis reaction box 19, and the hydrolysis reaction box 19 has a feed inlet. In order to be able to control the hydrogen production amount of hydrogen on-line according to the demand of the load (for example, a hydrogen fuel cell), to realize hydrogen production and controllable hydrogen at any time, the hydrogen production device also includes a feeding unit, and the feeding unit passes through the feed port The magnesium hydride is delivered into the hydrolysis reaction box 19, so that the magnesium hydride and the water in the hydrolysis reaction box 19 undergo a hydrolysis reaction to generate hydrogen. Magnesium hydride has a high hydrogen storage capacity, abundant sources of raw materials, and little impact on the environment. Therefore, in this embodiment, magnesium hydride is used as a raw material to produce hydrogen, which can well overcome the exi...
Embodiment 2
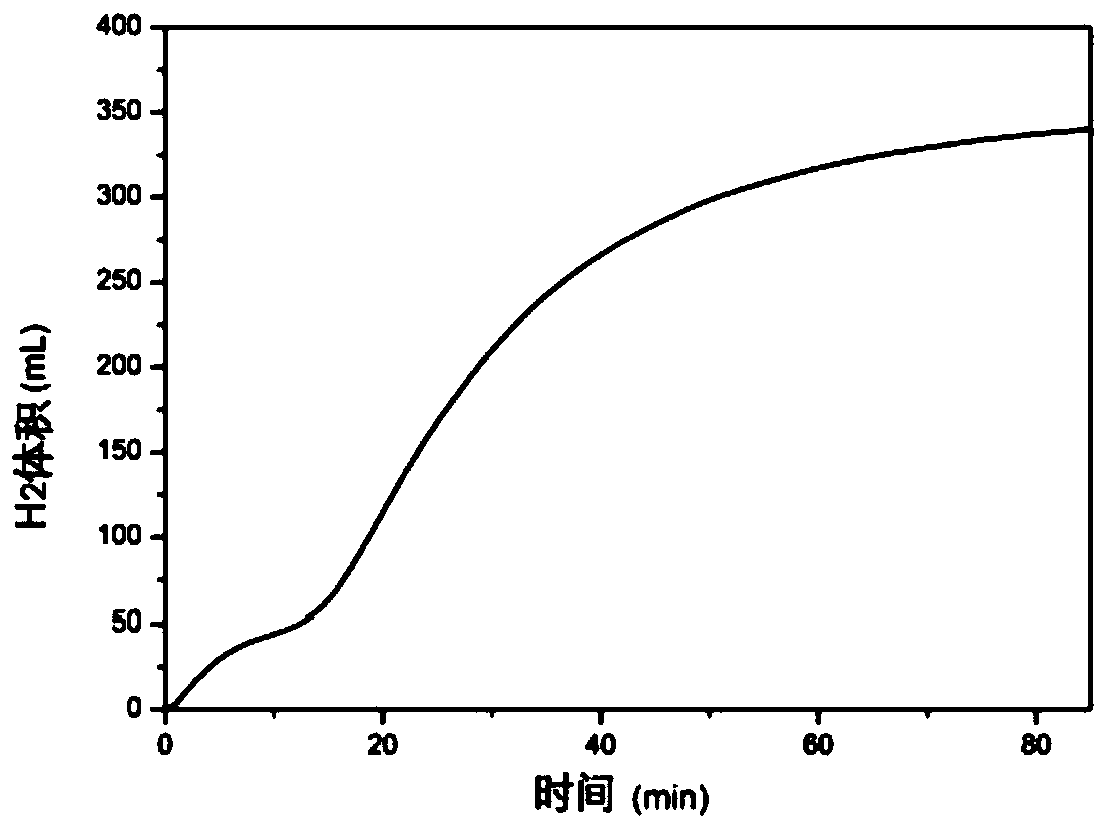
[0069] In the present embodiment, first input 0.5mol / L MgCl in the hydrolysis reaction box 2 solution, where Mg 2+ The ion concentration is 0.5mol / L, Cl - The concentration of ion is 1.0mol / L, then adds magnesium hydride in the hydrolysis reaction tank again, as figure 2 As shown, at the above ion concentration MgCl 2 Under the solution, the efficiency of hydrogen production by hydrolysis is close to 100%. The average hydrogen production rate for the first feeding is 4.7mL / min. The solution can be recycled 8 times. In order to ensure the stability of the hydrolysis rate, the preferred number of solution cycles is 3 times.
[0070] The MgCl 2 The principle that the solution can promote the reaction to produce hydrogen is:
[0071] MgCl 2 +2H 2 O→Mg(OH) 2aq ↓+2HCl (1)
[0072] MgH 2 +2HCl→MgCl 2 +2H 2 ↑ (2)
[0073] Mg(OH) 2su ↓+2HCl→MgCl 2 +2H 2 O (3)
[0074] That is, magnesium chloride and water react to form magnesium hydroxide and hydrochloric acid in the s...
Embodiment 3
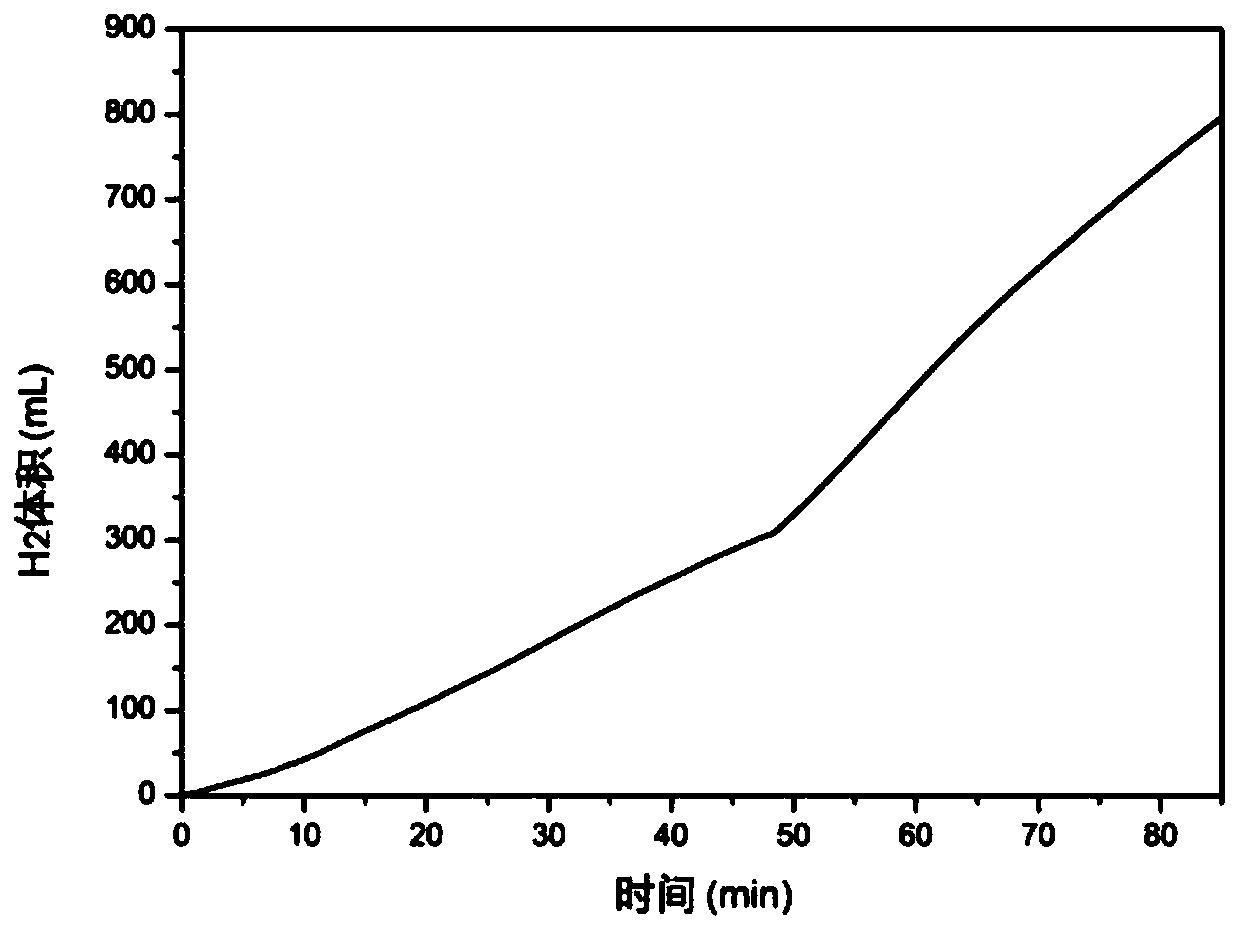
[0076] In the present embodiment, first input 0.5mol / L MgCl in the hydrolysis reaction box 2 solution and 0.05mol / L MgSO 4 solution, where Mg 2+ The concentration of ions is 0.55mol / L, Cl - The ion concentration is 1.0mol / L, SO 4 2- The concentration of ions is 0.05mol / L, and magnesium hydride is added in the hydrolysis reaction box afterwards, as image 3 As shown, at the above ion concentration MgCl 2 solution and MgSO 4 Under the mixed solution of the solution, the efficiency of hydrolysis hydrogen production is close to 98%, the average hydrogen production rate of the first feeding is 9.4mL / min, and the feeding amount of magnesium hydride reaches 0.5mol / L MgCl 2 2 times of the solution, the solution can be recycled 8 times, in order to ensure the stability of the hydrolysis rate, the preferred number of solution cycles is 3 times.
[0077] In this embodiment, the presence of a small amount of magnesium sulfate can improve the volume expansion of magnesium hydroxide ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| length | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com