Linear alkylbenzene production process and linear alkylbenzene production device
A technology for the production of straight-chain alkylbenzenes, which is applied in the production of bulk chemicals, chemical industry, sustainable manufacturing/processing, etc., and can solve the problems of increasing operational difficulty and energy consumption, short catalyst operation period, high reaction temperature and pressure, etc. problems, to achieve the effects of low production cost, easy regeneration and recycling, and high selectivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0109] Optionally, the preparation method of the solid acid catalyst comprises:
[0110] Add binder and 0.5-0.6mol / L HNO to the catalyst (molecular sieve) 3 Mix evenly to shape the catalyst, and the molding process is carried out according to the conventional method;
[0111] Place the shaped catalyst in an oven and dry it at 60-120°C for 1-10 hours, and then bake it in a muffle furnace at 400-700°C for 1-10 hours;
[0112] Under the pressure of 0.1-0.5MPa (g), use 100% water vapor (mass space velocity of water 1-6h) in the tubular fixed-bed reactor -1 ) treating the catalyst at 400-600° C. for 2-20 hours, and drying at 100-150° C. for 5-10 hours to obtain the solid acid catalyst used in the alkylbenzene production process of the present invention.
[0113] Usually, the alkylation reaction between linear olefins and benzene will produce various linear alkylbenzene isomers such as 2-LAB, 3-LAB, 4-LAB, 5-LAB and so on. Among them, 2-LAB has a long "tail" during sulfonation, w...
Embodiment 1
[0131] The linear olefins used in Example 1 are C8 linear olefins.
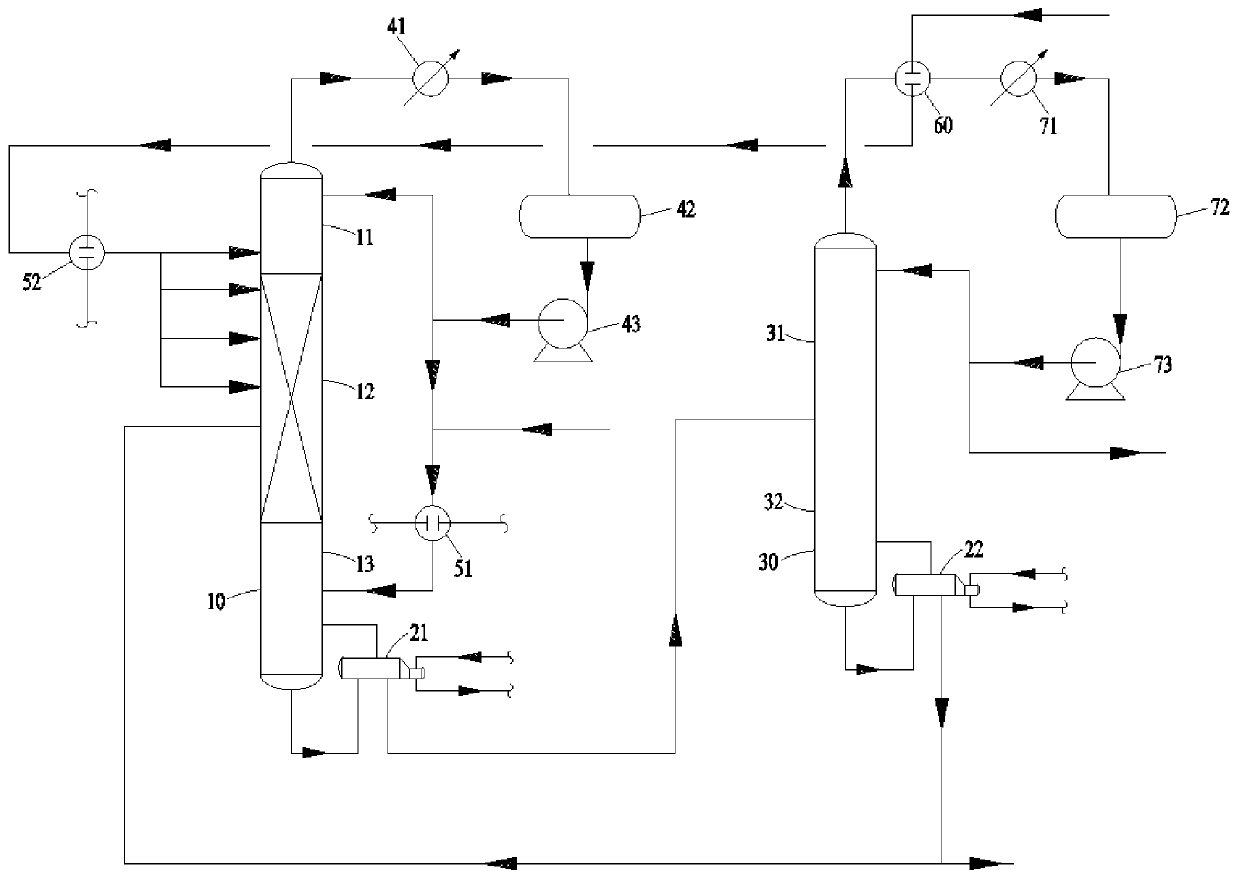
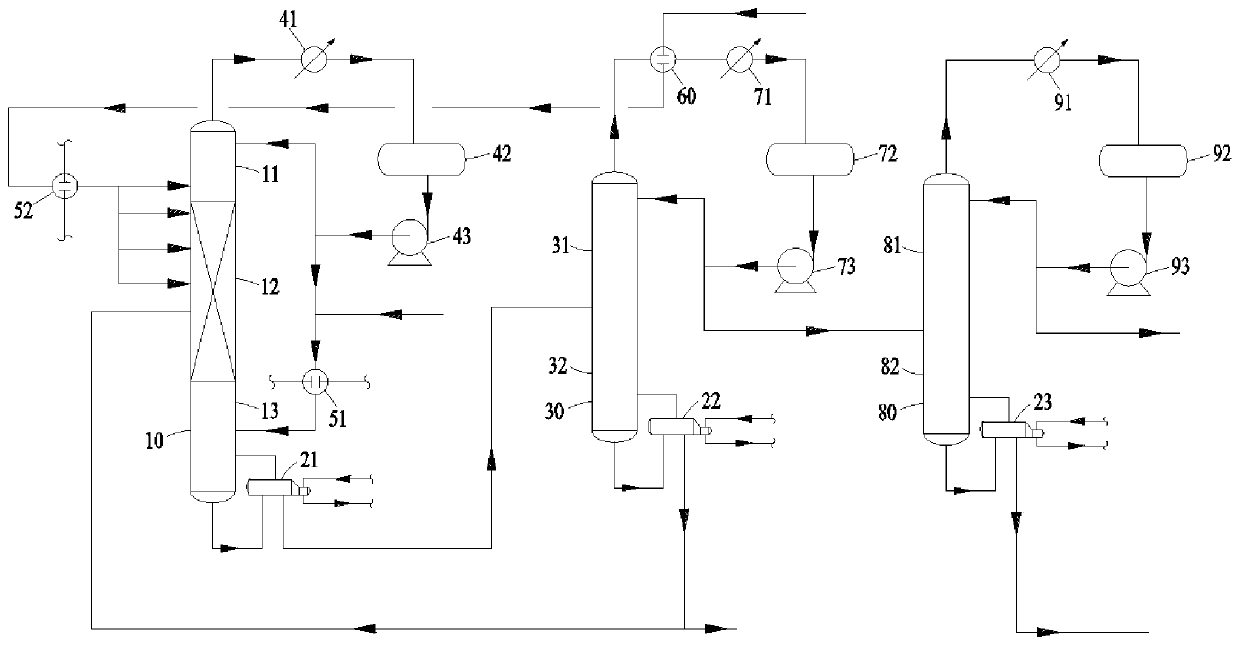
[0132] The inner diameter of the linear alkylbenzene reactive distillation tower 10 is 50mm, and the height is 3000mm, wherein the height of the first rectification section 11 is 150mm, and the height of the reaction section 12 is 1200mm, and the first stripping section The height of 13 is 1650mm.
[0133] During the entire alkylbenzene production process, the ratio of the total amount of benzene added to the linear alkylbenzene reactive distillation column 10 as production raw materials to the total amount of linear olefins is 1.05:1.
[0134] The reaction conditions and results are shown in Table 1. The results show that the mass proportion of C8 linear alkylbenzene in the reaction product reaches more than 95%, and in the linear alkylbenzene, the mass proportion of 2-LAB is greater than 50%. 3 -The mass proportion of LAB is greater than 35%.
[0135] Table 1 Example 1 reaction conditions and reaction res...
Embodiment 2
[0138] The linear olefins used in Example 2 are C12 linear olefins.
[0139] The inner diameter of the linear alkylbenzene reactive distillation tower 10 is 60mm, and the height is 3000mm, wherein the height of the first rectifying section 11 is 240mm, and the height of the reaction section 12 is 1260mm, and the first stripping section 13 has a height of 1500mm.
[0140] During the entire alkylbenzene production process, the ratio of the total amount of benzene added to the linear alkylbenzene reactive distillation column 10 as production raw materials to the total amount of linear olefins is 1.08:1.
[0141] The reaction conditions and results are shown in Table 2. The results show that the mass proportion of C12 linear alkylbenzene in the reaction product reaches more than 95%, and in the linear alkylbenzene, the mass proportion of 2-LAB is greater than 50%. -The mass proportion of LAB is greater than 35%.
[0142] Table 2 embodiment 2 reaction conditions and reaction resu...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com