A polyphenylene ether type anion exchange membrane loaded with tetraamino quaternary phosphonium cation units and its preparation method
A technology of tetraamino quaternary and phosphonium cations, applied in electrical components, electrochemical generators, fuel cells, etc. Structure, not involved in anion exchange membrane research and other issues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] Dissolve 1.9 g of brominated polyphenylene ether in 5 ml of chlorobenzene, add 0.1 g of an alkyl-substituted organic phosphine monomer shown in formula (2), and stir at room temperature for 5 hours to obtain a brown polyphenylene ether containing grafted quaternary phosphonium cations. solution; wherein m in the main chain of brominated polyphenylene ether is 0.55, wherein the values of x and y in the organic phosphine monomer are all 1, R 1 is methyl, R 2 and R 3 For n-hexyl.
[0039] Pour the obtained brown solution onto a clean and dry glass plate, and dry it at 60°C to form a film;
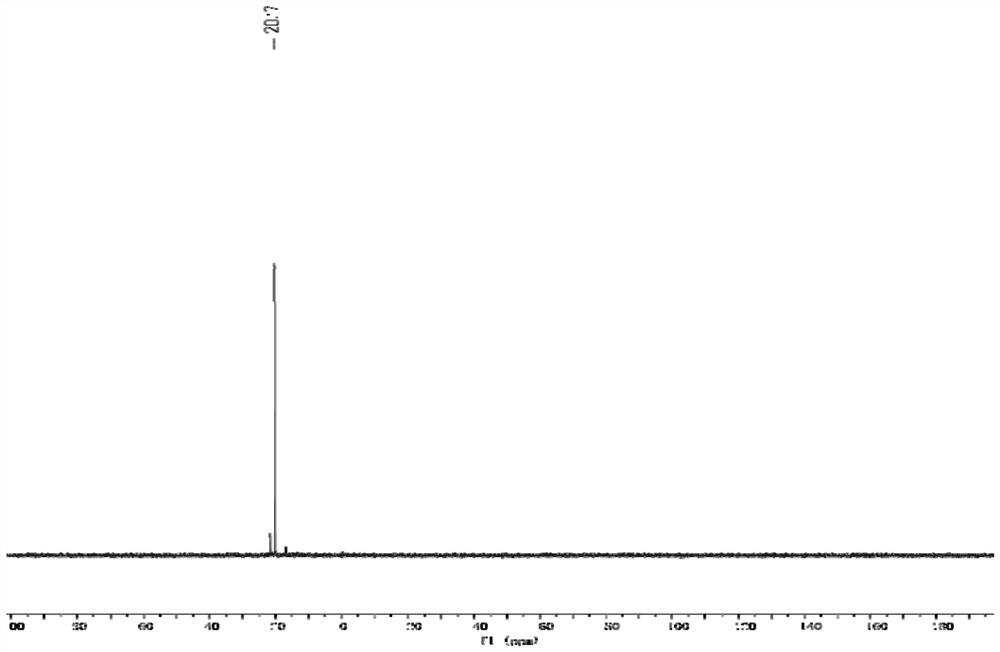
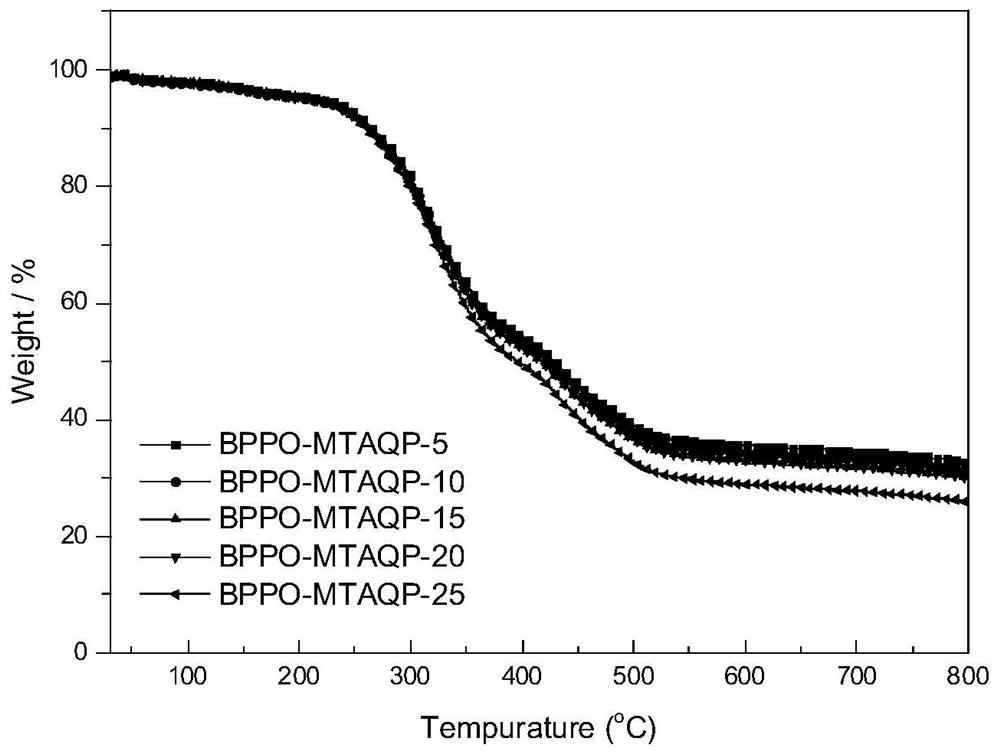
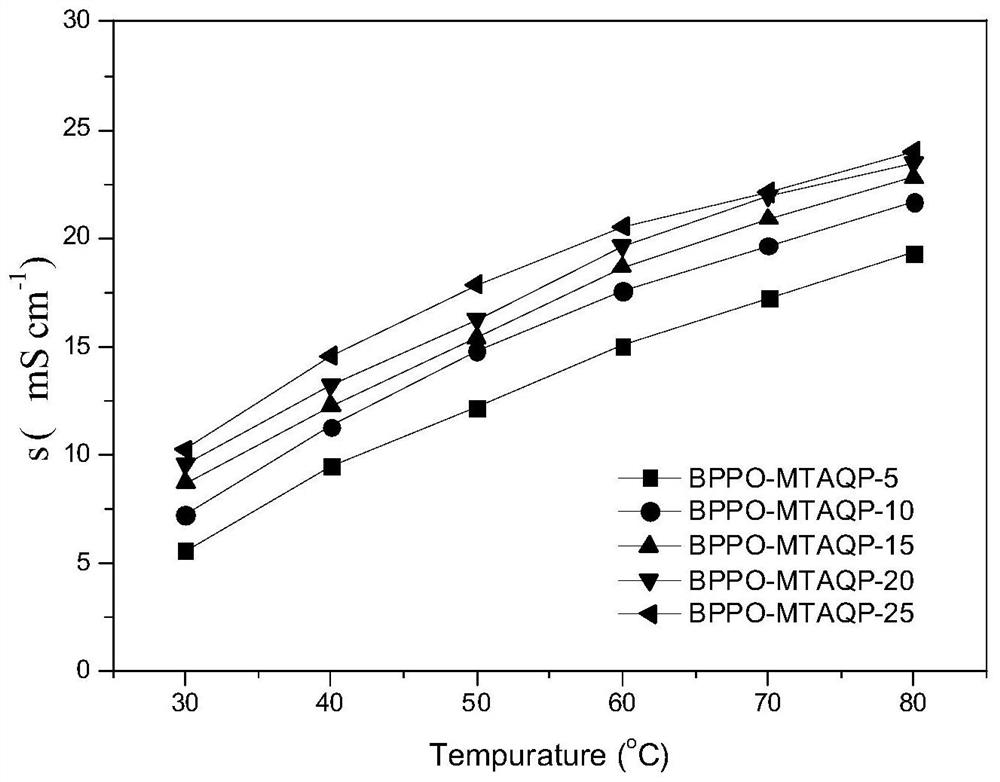
[0040] Soak the membrane in 1M NaOH solution for 48h for OH - For ion exchange, use deionized water to wash off the remaining NaOH on the surface of the membrane, and measure the ion exchange capacity by back titration to calculate the substitution ratio of quaternary phosphonium cations. For the NMR phosphorus spectrum of anion exchange membrane, see figure 1 , the thermogravimet...
Embodiment 2
[0042]Dissolve 1.8g of brominated polyphenylene ether in 5ml of chlorobenzene, add 0.2g of an alkyl-substituted organic phosphine monomer shown in formula (2), and stir for 5 hours at room temperature to obtain a brown polyphenylene ether containing grafted quaternary phosphonium cations. solution; wherein m in the main chain of brominated polyphenylene ether is 0.55, wherein the values of x and y in the organic phosphine monomer are all 1, R 1 is methyl, R 2 and R 3 For n-hexyl.
[0043] The obtained brown solution was poured on a clean and dry glass plate, and dried at 60°C to form a film;
[0044] Soak the membrane in 1M NaOH solution for 48h for OH - For ion exchange, the remaining NaOH on the surface of the membrane was washed with deionized water, and the ion exchange capacity was measured by back titration to calculate the substitution ratio of quaternary phosphonium cations. The anion exchange membrane with 10% organic phosphine monomer was named BPPO-MTAQP-10%, ...
Embodiment 3
[0046] Dissolve 1.7g of brominated polyphenylene ether in 5ml of chlorobenzene, add 0.3g of an alkyl-substituted organic phosphine monomer shown in formula (2), and stir for 5 hours at room temperature to obtain a brown polyphenylene ether containing grafted quaternary phosphonium cations. solution; wherein m in the main chain of brominated polyphenylene ether is 0.55, wherein the values of x and y in the organic phosphine monomer are all 1, R 1 is methyl, R 2 and R 3 For n-hexyl.
[0047] The obtained brown solution was poured on a clean and dry glass plate, and dried at 60°C to form a film;
[0048] Soak the membrane in 1M NaOH solution for 48h for OH - For ion exchange, the remaining NaOH on the surface of the membrane was washed with deionized water, and the ion exchange capacity was measured by back titration to calculate the substitution ratio of quaternary phosphonium cations. The anion-exchange membrane with an organic phosphine monomer feeding ratio of 15% was n...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com