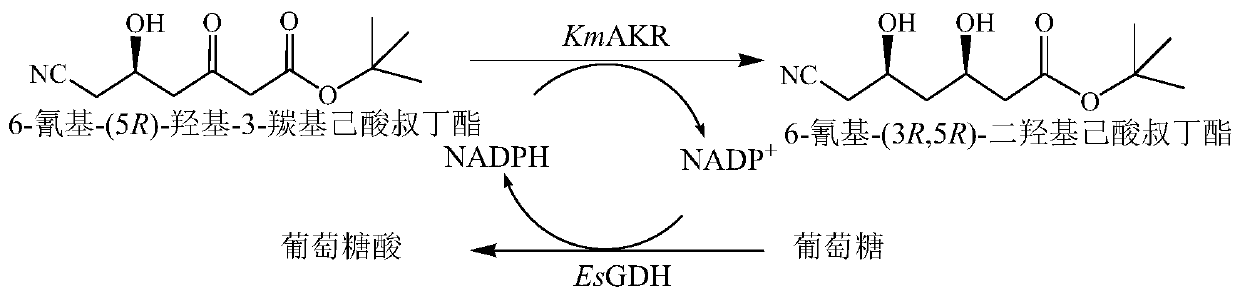
Recombinant aldo-keto reductase mutant and application thereof
A reductase and mutant technology, which is used in recombinant aldo-ketone reductase mutants and application fields, and can solve the problems of low substrate feeding amount and low asymmetric reduction activity.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] Example 1: Construction and screening of aldehyde and ketone reductase mutant library
[0023] The preparation of the aldehyde and ketone reductase mutant library was achieved by three rounds of site-directed saturation mutagenesis. The primers were designed as shown in Table 1. In the first round, E.coli BL21(DE3) / pET28a(+)-kmakr-W297H / Y296W was used for construction (see The vector pET28a(+)-kmakr-W297H / Y296W in the patent application 201710282633.X) was used as a template, Lys29-F and Lys29-R in Table 1 were used as primers, and the aldehyde and ketone shown in SEQ ID NO.2 were synthesized by saturation mutation PCR. The 29th lysine of the reductase KmAKR-W297H / Y296W amino acid sequence was mutated into the remaining 19 amino acids, and transformed, smeared on a plate, and obtained the aldehyde and ketone reductase mutant KmAKR-W297H / Y296W / K29H (ie SEQ The 29th lysine of the amino acid shown in ID NO.2 is mutated to histidine) and KmAKR-W297H / Y296W / K29R (that is, the...
Embodiment 2
[0028] Embodiment 2: Induced expression of control group aldehyde and ketone reductase, mutant and glucose dehydrogenase
[0029] Preparation of glucose dehydrogenase wet cells: the E.coli BL21(DE3) / pET28b(+)-esgdh is inserted into pET28b(+) by the glucose dehydrogenase gene (GenBank NO.KM817194.1) from Exiguobacterium sibirium DSM 17290 A recombinant expression vector was constructed, and the expression vector was transformed into E. coli BL21(DE3) to obtain E coli BL21(DE3) / pET28b(+)-esgdh.
[0030] The starting strain E.coli BL21(DE3) / pET28a(+)-kmakr-W297H / Y296W in Example 1 and the aldehyde and ketone reductase mutant strain screened in Example 1 and E coli BL21(DE3) / pET28b(+)-esgdh They were inoculated into LB liquid medium containing a final concentration of 50 μg / mL kanamycin, cultured at 37°C for 10 h, and inoculated with a volume concentration of 1.5% (v / v) into fresh LB liquid medium containing a final concentration of 50 μg / mL kanamycin. Cultivate in LB liquid medi...
Embodiment 3
[0031] Example 3: Mutant library screening
[0032] Mix the wet cells of the mutant strain induced and expressed in Example 2 and the wet cells of glucose dehydrogenase at a dry weight ratio of 3.5:1 (w / w) to form a mixed cell, add pH 7.0, 100mM PBS buffer to resuspend, Obtain the mixed bacterial liquid of mutant strains. Under the same conditions, the control strain E.coli BL21(DE3) / pET28a(+)-kmakr-W297H / Y296W was used to replace the wet cells of the mutant strain to prepare a mixed bacterial solution.
[0033] The mixed bacterial solution of the mutant strain and the mixed bacterial solution of the control strain were used as catalysts, tert-butyl 6-cyano-(5R)-hydroxy-3-carbonylhexanoate was used as the substrate, glucose was used as the auxiliary substrate, and no external derived NADPH or NADP + , using the endogenous NADPH of the bacteria to establish a coenzyme circulation system. The reaction system is selected as 10mL, the amount of catalyst is 25g / L based on the to...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com