Coding gene of phospholipase D and expression and application thereof
A phospholipase and encoding technology, which is applied in the fields of application, genetic engineering, plant gene improvement, etc., can solve the problems of long fermentation cycle and complicated culture conditions, and achieve short fermentation cycle, good stability of enzyme activity, and good acylation ability Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] The present invention provides a phospholipase D coding sequence (sspld), comprising 522 amino acids. The amino acid sequence is shown in SEQ ID NO. 1, and the gene encoding the phospholipase D has the nucleotides shown in SEQ ID NO. 2. The sequence is 1,566 nucleotides in length.
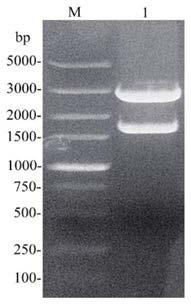
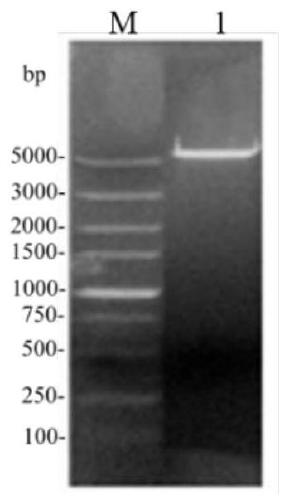
[0040] The present invention also provides a recombinant plasmid expressing phospholipase D, which includes the nucleotide sequence shown in SEQ ID NO. 2 and has a total length of 6916 nucleotides.
Embodiment 2
[0042] This example provides the construction of the recombinant plasmid pET-28a(+)-sspld and its expression method in E. coli BL21. The specific steps are as follows:
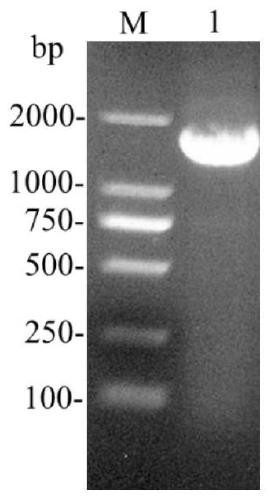
[0043] (1) Amplification of phospholipase D coding sequence
[0044] Using the phospholipase D coding sequence sspld from Streptomyces preserved in the laboratory as a template, design primers (P1, P2) to amplify the phospholipase D coding sequence:
[0045] Primer P1: 5’-CG AAGCTT ATGGCACGTCATCC-3’(Hind III)
[0046] Primer P2: 5’-CC GGATCC TTAGCCAGCCAGAT-3’(BamH I)
[0047] PCR amplification reaction is carried out in a 50μL system, and 25μL is added to the reaction system (Premix), 20μL ddH 2 O, 2μL of template DNA, 1.5μL of upstream and downstream primers. The reaction conditions were 30 cycles of pre-denaturation at 94°C for 3 minutes: denaturation at 94°C for 30 seconds, annealing at 52.4°C for 30 seconds, extension at 72°C for 1.5 minutes, and a final extension at 72°C for 10 minutes. The PCR products are i...
Embodiment 3
[0057] This embodiment provides a culture method for increasing the activity of phospholipase D through induction culture.
[0058] (1) The influence of induction temperature on enzyme production
[0059] When other fermentation induction conditions are the same, the recombinant bacteria are placed at 15, 20, 25, 30, 35 ℃ and other different temperature conditions for 8 hours after induction, according to the standard activity determination method to determine the phospholipase D under different induction temperature conditions Enzyme activity. by Figure 7 a It can be seen that the fermentation enzyme activity is highest when the induction temperature is 20℃.
[0060] (2) The effect of induction timing on enzyme production
[0061] The recombinant bacteria were cultured to OD600nm of 0.6, 0.8, 1.0, 1.2, 1.4 for IPTG induction, the induction time remained the same, and the enzyme activity of phospholipase D under different induction time conditions was measured according to the stand...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com