Method for preparation of nickel-cobalt-manganese ternary material with aluminum oxide coating layer by taking retired lithium ion battery as raw material
A technology of lithium-ion batteries and ternary materials, applied to electrical components, secondary batteries, battery electrodes, etc., can solve problems such as waste, secondary pollution of aluminum resources, and problems not involving the treatment of aluminum-containing alkali immersion solutions, and achieve High structural stability, strong cycle performance, and the effect of avoiding waste of aluminum resources
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
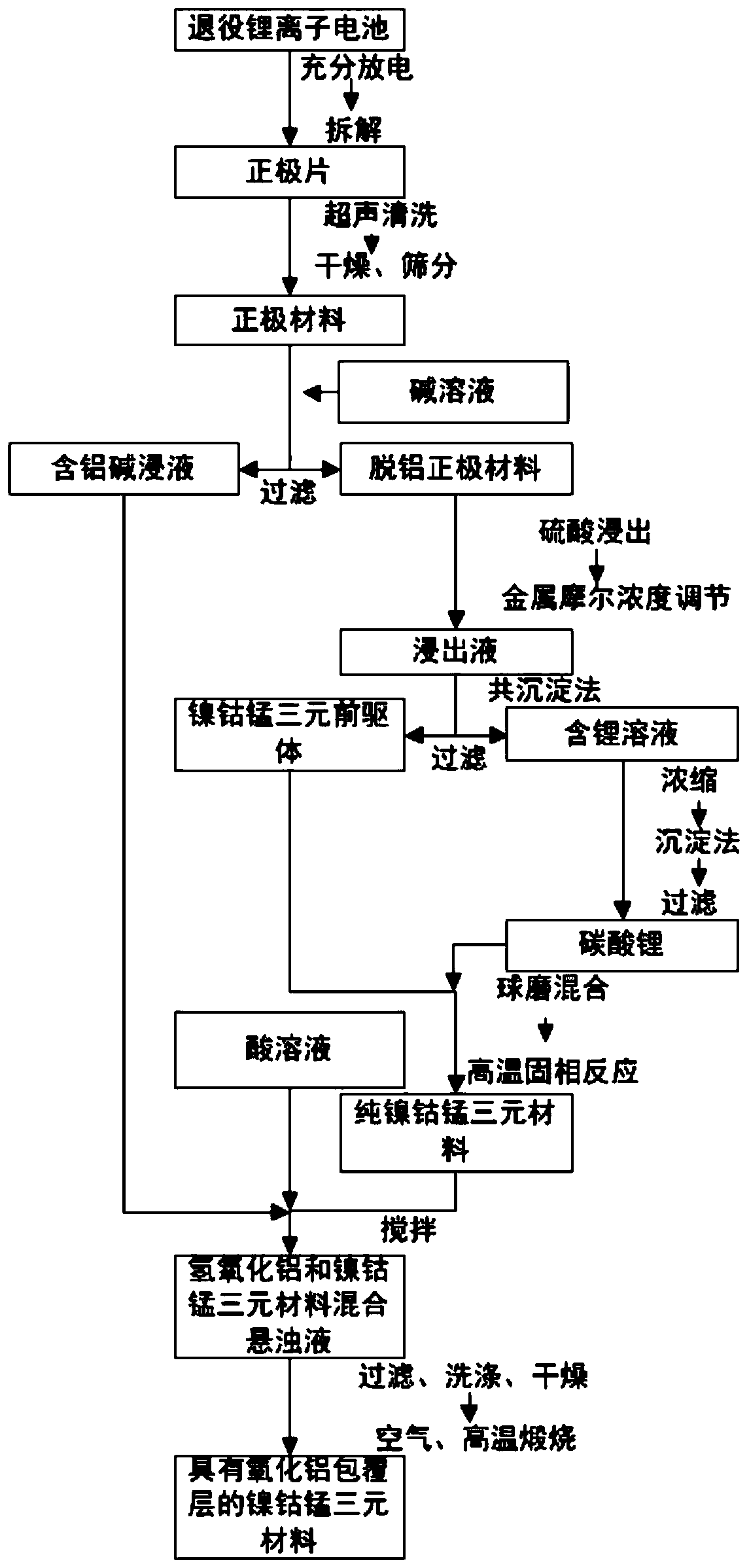
[0040] refer to figure 1 , a method for preparing a nickel-cobalt-manganese ternary material with an aluminum oxide coating layer using decommissioned lithium-ion batteries as raw materials of the present invention, comprising:
[0041] S100, dismantling the decommissioned lithium ion battery to obtain a positive electrode material containing nickel, cobalt and manganese;
[0042] S200, mixing the positive electrode material with an alkaline solution for alkaline leaching, and filtering to obtain an aluminum-containing alkaline immersion solution and a dealuminated positive electrode material;
[0043] S300, preparing a pure nickel-cobalt-manganese ternary material by using the above-mentioned dealuminated cathode material as a raw material;
[0044] S400, mixing the above-mentioned pure nickel-cobalt-manganese ternary material with the above-mentioned aluminum-containing alkali immersion solution, adding an acid solution and stirring to obtain a mixed suspension of aluminum ...
Embodiment 2
[0065] A method for preparing a nickel-cobalt-manganese ternary material with an aluminum oxide coating layer from decommissioned lithium-ion batteries in Example 2 of the present invention is basically the same as in Example 1.
[0066] specific:
[0067] In step S100, the specific operation is: fully discharge the decommissioned nickel-cobalt-manganese ternary lithium-ion battery, and disassemble the positive electrode sheet containing nickel-cobalt-manganese element, and then perform ultrasonic cleaning and stripping on the above-mentioned positive electrode sheet to obtain aluminum foil and The mixture of positive electrode materials is finally dried and sieved to obtain positive electrode materials.
[0068] In step S200, the solute in the alkaline solution is sodium hydroxide; the concentration of the alkaline solution is 0.1mol / L; the solid-liquid ratio of the positive electrode material to the alkaline solution is 100g / L, and the alkaline immersion temperature is 40°C....
Embodiment 3
[0081] A method for preparing a nickel-cobalt-manganese ternary material with an aluminum oxide coating layer from decommissioned lithium-ion batteries in Example 3 of the present invention is basically the same as in Example 1.
[0082] specific:
[0083] In step S100, the specific operation is: fully discharge the decommissioned nickel-cobalt-manganese ternary lithium-ion battery, and disassemble the positive electrode sheet containing nickel-cobalt-manganese element, and then perform ultrasonic cleaning and stripping on the above-mentioned positive electrode sheet to obtain aluminum foil and The mixture of positive electrode materials is finally dried and sieved to obtain positive electrode materials.
[0084] In step S200, the solute in the alkaline solution is sodium hydroxide; the concentration of the alkaline solution is 0.5mol / L; the solid-liquid ratio of the positive electrode material to the alkaline solution is 200g / L, and the alkaline immersion temperature is 50°C....
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com