A novel nitrogen-heterofused ring compound and its preparation method and application
A compound and fused ring technology, which is applied in the field of new nitrogen heterofused ring compounds and their preparation, can solve the problems of unsatisfactory material performance and stability, and achieve the advantages of electron transmission, good stability and solubility, and increased Solubility effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0055] A preparation method of a novel nitrogen-heterofused ring compound, comprising the steps of:
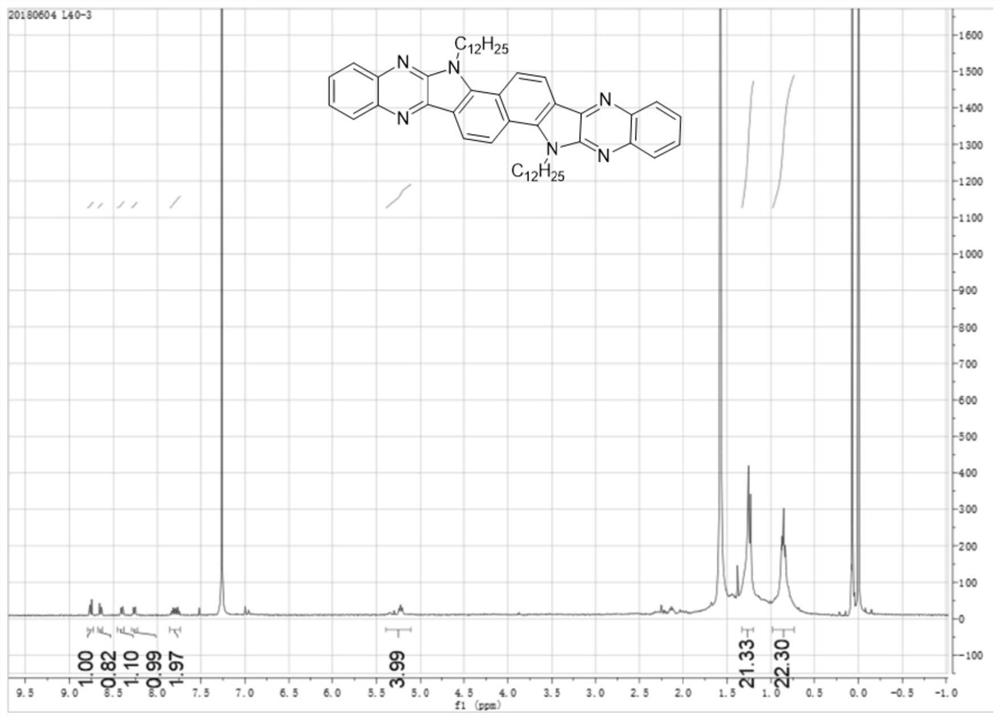
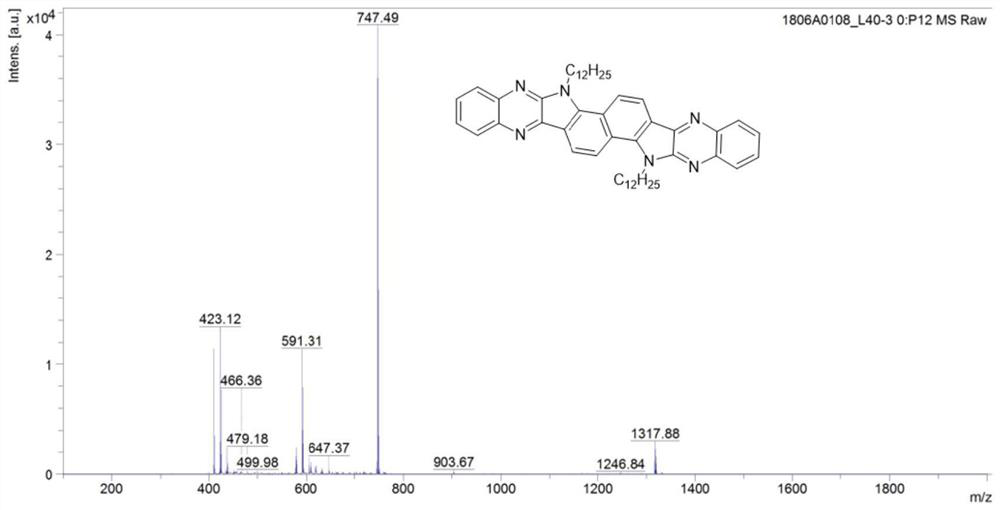
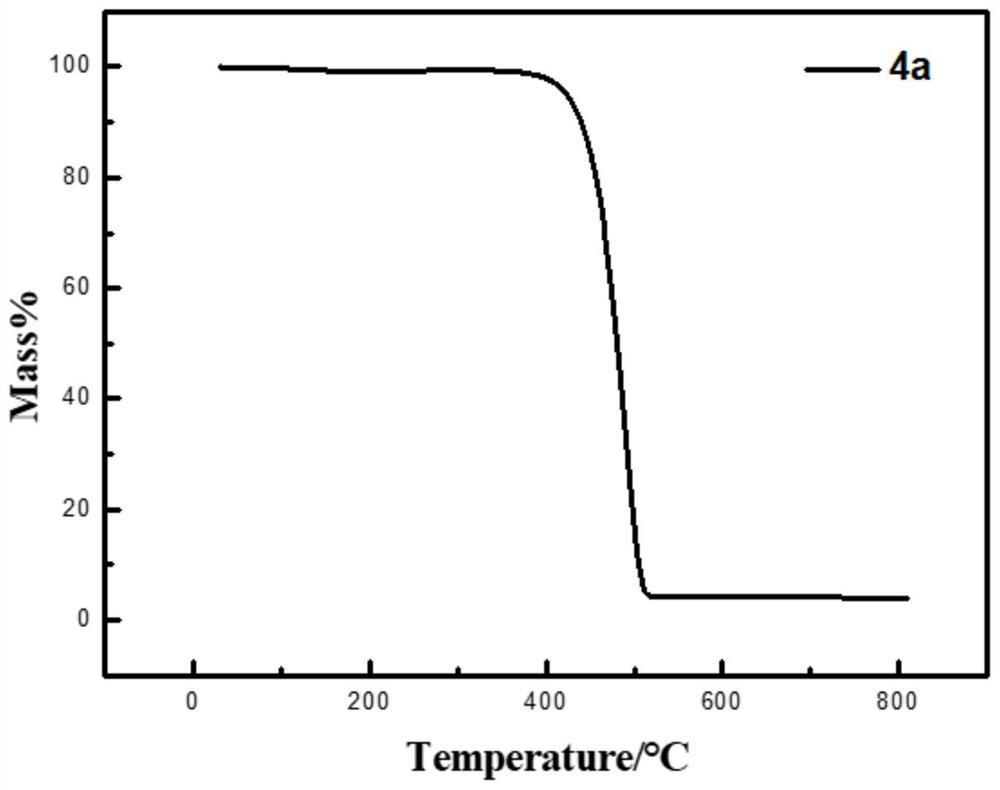
[0056] Under nitrogen atmosphere, mix 38mg 1,2 phenylenediamine (compound I) with 106mg 3,8-bis(2-dodecyl)-3,8-dihydroindole[7,6g]indole-1 , 2,6,7-tetraketone (compound II) was added to a single-necked flask (equivalent ratio of 2 to 1), and then 8 mL of glacial acetic acid was added to react at 120 ° C for 12 h. The obtained solution was distilled under reduced pressure to remove acetic acid, and then purified by a chromatographic column. The eluent was dichloromethane. After separation and purification, the dichloromethane was distilled under reduced pressure and spin-dried to obtain a yellow solid product (compound III). Mass 48.7 mg, yield 37%. of the product 1 H NMR spectrum as figure 1 As shown, the mass spectrum is as figure 2 As shown, the thermogravimetric analysis diagram is shown as image 3 As shown, the spectrum can prove that compound III has good thermal s...
Embodiment 2
[0061] A preparation method of a novel nitrogen-heterofused ring compound, comprising the steps of:
[0062] Under nitrogen atmosphere, mix 20mg 1,2 naphthalene diamine (compound IV) with 52mg 3,8-bis(2-octyldodecyl)-3,8-dihydroindoline[7,6g]indole -1,2,6,7-tetraketone (compound V) was added into a single-necked flask (equivalent ratio of 2 to 1), and then 4 mL of glacial acetic acid was added, and reacted at 120°C for 4 h. The obtained solution was distilled under reduced pressure to remove acetic acid first, and then purified by a chromatographic column. The eluent was toluene. After separation and purification, the toluene was distilled under reduced pressure and spin-dried toluene to obtain a red solid product (compound VI). The obtained compound quality was 30 mg. The yield was 43.8%. of the product 1 H NMR spectrum as Figure 4 As shown, the mass spectrum is as Figure 5 As shown, the thermogravimetric analysis diagram is shown as Image 6 As shown, the spectrum can...
Embodiment 3
[0067] A preparation method of a novel nitrogen-heterofused ring compound, comprising the steps of:
[0068] Under nitrogen atmosphere, mix 8.7mg 4,5 difluoro-1,2 phenylenediamine (compound A) with 20 mg 3,8-bis(2-octyldodecyl)-3,8-dihydroindoline [7,6g] Indole-1,2,6,7-tetraketone (compound V) was added into a one-necked flask, then 2mL glacial acetic acid and 2mL chloroform were added, and the reaction was carried out at 75°C for 1.5h. The obtained solution was distilled off under reduced pressure to remove the solvent, and then purified by a chromatographic column. The eluent was dichloromethane. After separation and purification, the dichloromethane was distilled under reduced pressure and spin-dried to obtain a yellow solid product (compound B). Mass 11.2 mg, yield 45%. of the product 1 H NMR spectrum as Figure 7 shown.
[0069] The chemical reaction equation of the above reaction is as follows:
[0070]
[0071] Wherein compound V was prepared by literature metho...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com